Potassium hexafluorozirconate (IV)
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
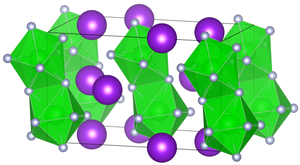
|
|||||||||||||||||||
| __ K + __ Zr 4+ __ F - | |||||||||||||||||||
| Crystal system |
monoclinic |
||||||||||||||||||
| Space group |
C 2 / c (No. 15) |
||||||||||||||||||
| Lattice parameters |
a = 6.57 (2) Å, b = 11.44 (2) Å, c = 6.94 (2) Å, β = 90.3 ° |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Potassium hexafluorozirconate (IV) | ||||||||||||||||||
| other names |
Dipotassium hexafluorozirconate (IV) |
||||||||||||||||||
| Ratio formula | K 2 [ZrF 6 ] | ||||||||||||||||||
| Brief description |
odorless, white crystalline solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 283.41 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
3.48 g cm −3 |
||||||||||||||||||
| solubility |
15.5 g l −1 in water (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Potassium hexafluorozirconate (IV) , K 2 [ZrF 6 ] is a chemical compound of potassium from the group of hexafluorozirconates .
properties
Potassium hexafluorozirconate (IV) is an odorless, white crystalline solid. It has a density of 3.48 g · cm −3 . The compound crystallizes - contrary to earlier assumptions not orthorhombically - in the monoclinic space group C 2 / c (space group no. 15) with the lattice constants a = 6.57 (2) Å, b = 11.44 (2) Å, c = 6.94 (2) Å and β = 90.3 °. There are four formula units in a unit cell.
safety instructions
The salt is toxic when taken orally. It causes irritation to the skin, respiratory tract and eyes. In studies on mice administered with potassium hexafluorozirconate (IV), hepatitis , acute pulmonary edema and spasticity of the lungs were observed.
Glass and strong oxidizing agents can cause violent reactions with the compound.
Individual evidence
- ↑ a b c data sheet Potassium hexafluorozirconate from AlfaAesar, accessed on March 5, 2019 ( PDF )(JavaScript required) .
- ↑ a b Entry on dipotassium hexafluorozirconate in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c d data sheet Potassium hexafluorozirconate from Sigma-Aldrich , accessed on March 5, 2019 ( PDF ).
- ^ R. Hoppe, B. Mehlhorn: The crystal structure of K 2 ZrF 6 . In: Journal of Inorganic and General Chemistry . tape 425 , no. 3 , September 1976, p. 200-208 , doi : 10.1002 / zaac.19764250303 .


