Potassium tetrachloridoplatinate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium tetrachloridoplatinate | |||||||||||||||
| other names |
|
|||||||||||||||
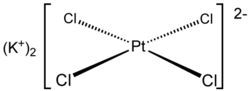
| Molecular formula | K 2 [PtCl 4 ] | |||||||||||||||
| Brief description |
red odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 415.09 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.4 g cm −3 |
|||||||||||||||
| Melting point |
500 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Potassium tetrachloridoplatinate (II) is a chemical compound from the group of tetrachloridoplatinates .
Extraction and presentation
Potassium tetrachloridoplatinate can be obtained by reacting potassium hexachloroplatinate (IV) with hydrazine salts such as hydrazine dihydrochloride .
properties
Potassium tetrachloridoplatinate is a red, odorless solid that is sparingly soluble in water. It has a tetragonal crystal structure with the space group P 4 / mmm (space group no. 123) and the lattice parameters a = 699 pm and c = 413 pm. The ammine complexes, [Pt (NH 3 ) 4 ] [PtCl 4 ] and [Pt (NH 3 ) 4 ] Cl 2 are formed with ammonia solution .
use
Potassium tetrachloridoplatinate is used for the production of metal bis (dithiolates), which are used, for example, in laser technology and optics. It is also used to produce colloidal platinum nanoparticles for catalysis and photocatalysts.
Individual evidence
- ↑ a b c d e f record with potassium tetrachloroplatinate in the GESTIS database of IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b c data sheet Potassium tetrachloroplatinate (II), 99.99% trace metals basis from Sigma-Aldrich , accessed on August 29, 2013 ( PDF ).
- ↑ a b c Georg Brauer: Handbook of preparative inorganic chemistry . 3., reworked. Edition. tape III . Enke, Stuttgart 1981, ISBN 3-432-87823-0 , pp. 1712 .
- ↑ Entry on Dipotassium tetrachloroplatinate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .



![{\ displaystyle \ mathrm {2 \ K_ {2} [PtCl_ {6}] + N_ {2} H_ {6} Cl_ {2} \ longrightarrow 2 \ K_ {2} [PtCl_ {4}] + 6 \ HCl + N_ {2} \ uparrow}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/503490f3689125453d1c75511268d4443d881737)
