Potassium hexachloroplatinate (IV)
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium hexachloroplatinate (IV) | |||||||||||||||
| other names |
Dipotassium hexachloroplatinate |
|||||||||||||||
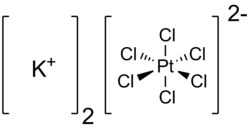
| Molecular formula | K 2 [PtCl 6 ] | |||||||||||||||
| Brief description |
yellow to orange-yellow odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 486.01 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.50 g cm −3 |
|||||||||||||||
| Melting point |
250 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.825 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Potassium hexachloroplatinate (IV) is a chemical compound from the group of hexachloridoplatinates .
Extraction and presentation
Potassium hexachloroplatinate (IV) can be obtained by reacting a weakly hydrochloric acid hexachloroplatinate (IV) acid solution or platinum (IV) chloride with potassium chloride .
properties
Potassium hexachloroplatinate (IV) is a yellow, odorless solid that is sparingly soluble in cold water. It is in the form of bright yellow octahedra . A discoloration of ocher yellow and reddish yellow indicates the presence of iridium , ruthenium and palladium . Even small amounts of rhodium impurities cause a green-yellow to green color cast. The connection has a cubic crystal structure with the space group Fm 3 m (space group no. 225) .
It decomposes when heated, reacts with potassium hydroxide to form platinum hydroxide complexes and is reduced to potassium tetrachloroplatinate (II) by copper (I) chloride .
use
Potassium hexachloroplatinate (IV) is used in the production of other chemical compounds (such as the drug ormaplatin ) and in analog photography .
The compound can (with certain restrictions) be used to detect potassium .
The compound is also used as the standard for the platinum-cobalt color number .
Individual evidence
- ↑ a b c d e f g h i data sheet potassium hexachloroplatinate (IV), Pt 39.6% from AlfaAesar, accessed on August 23, 2013 ( PDF )(JavaScript required) .
- ↑ a b c Entry on potassium hexachloroplatinate in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b Data sheet potassium hexachloroplatinate (IV) (PDF) from Merck , accessed on August 23, 2013.
- ↑ Entry on Dipotassium hexachloroplatinate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b c R.D. Madan: Advanced Inorganic Chemistry . S. Chand, 2005, ISBN 81-219-1787-5 , pp. 426 ( limited preview in Google Book search).
- ↑ a b Georg Brauer: Handbook of preparative inorganic chemistry . 3., reworked. Edition. tape III . Enke, Stuttgart 1981, ISBN 3-432-87823-0 , pp. 1712 .
- ^ Franz von Bruchhausen, Hermann Hager, Siegfried Ebel, Eberhard Hackenthal, Ulrike Holzgrabe: Hager's Handbook of Pharmaceutical Practice: Substances LZ . Springer DE, 1999, ISBN 3-642-58388-1 , p. 356 ( limited preview in Google Book Search).
- ↑ by Jean-Louis Burgot: Ionic Equilibria in Analytical Chemistry - Jean-Louis Burgot . Springer, 2012, ISBN 1-4419-8381-3 , pp. 562 ( limited preview in Google Book search).
- ^ Helmut Hofmann, Gerhart Jander: Qualitative analysis . Walter de Gruyter, 1972, ISBN 3-11-003653-3 , p. 93 ( limited preview in Google Book search).



![{\ displaystyle \ mathrm {H_ {2} [PtCl_ {6}] + 2 \ KCl \ longrightarrow K_ {2} [PtCl_ {6}] \ downarrow +2 \ HCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/82e7a780931c6c25e3e446368874cb6fc07ac761)
![{\ displaystyle \ mathrm {PtCl_ {4} +2 \ KCl \ longrightarrow K_ {2} [PtCl_ {6}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c9c7b0cb1fd6667860591968e9366054f9697e0f)
![{\ displaystyle \ mathrm {K_ {2} [PtCl_ {6}] \ longrightarrow Pt + 2 \ KCl + 2 \ Cl_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4e44277c507b92cf1c5a9fb0b98282d7775fc56a)
![{\ displaystyle \ mathrm {K_ {2} [PtCl_ {6}] + 6 \ KOH \ longrightarrow K_ {2} [Pt (OH) _ {6}] + 6 \ KCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/91fdd0a32d7e8d94b82ffc44d10f4e1b0976bd35)
![{\ displaystyle \ mathrm {K_ {2} [PtCl_ {6}] + 2 \ CuCl \ longrightarrow K_ {2} [PtCl_ {4}] + 2 \ CuCl_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cbd8f50fe2887c9309a08058ab9a5be3000f7198)