Katanosine
| Katanosine | |||
| Surname | Katanosin A | Katanosin B | |
| Structural formula | 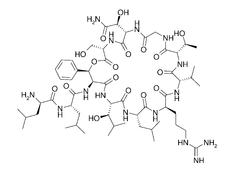 |

|
|
| other names | Lysobactin | ||
| CAS number | 116103-86-7 | 116340-02-4 | |
| PubChem | 3082767 | 11693839 | |
| Molecular formula | C 57 H 95 N 15 O 17 | C 58 H 97 N 15 O 17 | |
| Molar mass | 1262.48 g mol −1 | 1276.51 g mol −1 | |
| Physical state | firmly | ||
|
GHS labeling |
|
||
| H and P phrases | see above | ||
| see above | |||
As katanosin refers to a group of antibiotics (also Lysobactin -antibiotics called). These are natural substances with a strong antibacterial effect. Katanosin A and Katanosin B (lysobactin) have been described so far.
Occurrence
Katanosins are obtained from the fermentation medium of microorganisms . The biotechnological extraction from Cytophaga and from the Gram-negative bacterium Lysobacter sp. reported in Japan and the United States.
structure
Katanosins are depsipeptides . The katanosins are not regular peptides of the primary metabolism , rather they originate from the bacterial secondary metabolism , which also allows the incorporation of unusual, non-proteinogenic amino acids . The non-ribosomal amino acids 3-hydroxy leucine , 3-hydroxy phenylalanine , 3-hydroxy asparagine and allo- threonine are found in the katanosines .
All katanosins have a cyclic part and a linear tail. The ring is closed by an ester bond (these are cyclic depsipeptides , more precisely acylcyclodepsipeptides).
Katanosin A and B differ at the amino acid position 7. The secondary metabolite Katanosin A carries a valine , the main metabolite Katanosin B carries an isoleucine .
Biological effect
The katanosins disrupt the bacterial cell wall biosynthesis. They are highly potent against dreaded gram-positive hospital germs such as B. against staphylococci and enterococci . Because of their promising effects, biological and chemical research groups have studied the katanosin antibiotics. The in vitro level of activity of katanosins is comparable to that of vancomycin .
Chemical synthesis
In 2007 the first two syntheses of katanosin B (lysobactin) were described.
swell
- ^ DP Bonner, J. O´Sullivan, SK Tanaka, JM Clark, RR Whitney, J Antibiot . 1988 , 41 , 1745-1751 PMID 3209466
- ↑ J. O´Sullivan, JE McCullough, AA Tymiak, DR Kirsch, WH Trejo, PA Prinicipe, J. Antibiot. 1988 , 41 , 1740-1744 PMID 3209465
- ↑ JI Shoji, H. Hinoo, K. Matsumoto, T. Hattori, T. Yoshida, S. Matsuura, E. Kondo, J. Antibiot. 1988 , 41 , 713-718 PMID 3403364
- ↑ F. von Nussbaum, S. Anlauf, J. Benet-Buchholz, D. Häbich, J. Köbberling, L. Musza, J. Telser, H. Rübsamen-Waigmann, NA Brunner Angew. Chem. 2007 , 119 , 2085-2088; Angew. Chem. Int. Ed. 2007 , 46 , 2039-2042 PMID 17211904
- ↑ A. Guzman-Martinez, R. Lamer, MS VanNieuwenhze, J. Am. Chem. Soc. 2007 , 129 , 6017-6021 PMID 17432854