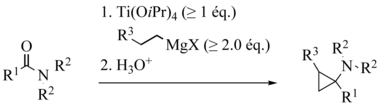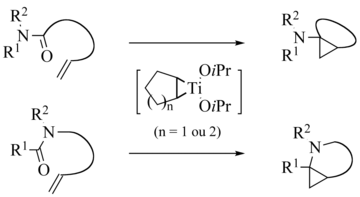Kulinkovich reaction
The Kulinkovich reaction is a name reaction in organic chemistry for the production of cyclopropanol derivatives. It was developed in 1989 by Kulinkovich et al. developed.
Overview
A carboxylic acid ester , a Grignard compound and a titanium (IV) alcoholate serve as starting materials for the Kulinkovich reaction . Titanium reagents with a hydrogen atom in the β position are formed in situ . The titanium reagent can be used in catalytic amounts.
For example, ClTi (OiPr) 3 , Ti (OiPr) 4 , ClTi (OtBu) 3 , Ti (OtBu) 4 can be used as titanium catalysts . Typical solvents are Et 2 O, THF or toluene. Tolerated functional groups are ethers R – O – R, thioethers R – S – R and imines RN = CHR. Amides , primary and secondary amines and most carbamates are not tolerated under the usual reaction conditions.
Reaction mechanism
The widely accepted reaction mechanism is based on two successive transmetalations with Grignard reagents . The resulting titanium complex disproportionates to an alkane and titanacyclopropane 1 . The insertion of the carbonyl group of the ester into the weakest carbon-titanium bond gives the oxatitanium acyclopentane 2 , which can then rearrange to the ketone 3 . Finally, the carbonyl group of 3 pushes again into the remaining carbon-titanium bond, whereby the cyclopropanol 4 is formed. The stabilization of the transition state of this step, which is also the rate-limiting one, by an agostic interaction of β-hydrogen and titanium is assumed to be the reason for the observed diastereoselectivity. Transmetalation of 4 leads to the largely observed product 5 - a magnesium alcoholate, from which the desired cyclopropoxide is formed by hydrolysis .
de Meijere variant
Armin de Meijere et al. developed a variant with amides instead of esters , whereby the main product is now an aminocyclopropane:
Intramolecular variants are also known:
Szymoniak variant
In the by Szymoniak et al. developed variant, the substrate is a nitrile and the main product is a cyclopropane with a primary amine group:
The reaction mechanism is analogous to that of the normal Kulinkovich reaction.
Individual evidence
- ^ OG Kulinkovich, SV Sviridov, DA Vasilevskii, TS Pritytskaya: Zh. Org. Khim. 25, 1989, p. 2244.
- ^ SV Sviridov, DA Vasilevski: Synthesis . 1991, p. 234.
- ↑ OG Kulinkovich, A. de Meijere: Chem. Rev. 100, 2000, p. 2789.
- ↑ F. Sato, H. Urabe, Okamoto: S. Chem. Rev. 100, 2000, p. 2835.
- ↑ Y.-D. Wu, Z.-X. Yu: J. Am. Chem. Soc. 123, 2001, p. 5777. doi: 10.1021 / ja010114q
- ^ OG Kulinkovich Russ. Chem. Bull. No. 5, 2004, pp. 1022-1043.
- ^ A. Wolan, Y. Six: Tetrahedron . 66, 2010, pp. 15-61.
- ^ A. Wolan, Y. Six: Tetrahedron . 66, 2010, pp. 3097-3133.
- ^ A b V. Chaplinski, A. de Meijere: Angew. Chem. Int. Ed. Engl. 35, 1996, pp. 413-414.
- ↑ A. de Meijere , H. Winsel, B. Stecker: Facile Synthesis of Aminocyclopropanes: N, N-Dibenzyl-N- (2-ethenylcyclopropyl) amine In: Organic Syntheses . 81, 2005, p. 14, doi : 10.15227 / orgsyn.081.0014 ( PDF ).
- ↑ J. Lee, JK Cha: J. Org. Chem. 62, 1997, pp. 1584-1585.
- ↑ V. Chaplinski, H. Winsel, M. Kordes, A. de Meijere: Synlett . 1997, pp. 111-114.
- ↑ B. Cao, D. Xiao, MM Joullié : Org. Lett. 1, 1999, pp. 1799-1801.
- ^ HB Lee, MJ Sung, SC Blackstock, JK Cha: J. Am. Chem. Soc. 123, 2001, pp. 11322-13324.
- ↑ M. Gensini, SI Kozhushkov, DS Yufit, JAK Howard, M. Es-Sayed, A. de Meijere: Eur. J. Org. Chem. 2002, pp. 2499-2507.
- ↑ G.-D. Tebben, K. Rauch, C. Stratmann, CM Williams, A. de Meijere: Org. Lett. 5, 2003, pp. 483-485.
- ↑ N. Ouhamou, Y. Six: Org. Biomol. Chem. 1, 2003, pp. 3007-3009.
- ↑ M. Gensini, A. de Meijere: Chem. Eur. J. 10, 2004, pp. 785-790.
- ↑ L. Larquetoux, JA Kowalska, Y. Six: Eur. J. Org. Chem. 2004, pp. 3517-3525.
- ↑ L. Larquetoux, N. Ouhamou, A. Chiaroni, Y. Six: Eur. J. Org. Chem. 2005, pp. 4654-4662.
- ↑ P. Bertus, J. Szymoniak: Chem. Commun. 2001, pp. 1792-1793.