Digerman
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
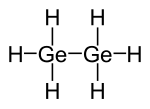
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Digerman | |||||||||||||||
| Molecular formula | Ge 2 H 6 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 151.33 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.98 g cm −3 (−109 ° C) |
|||||||||||||||
| Melting point |
−109 ° C |
|||||||||||||||
| boiling point |
31.5 ° C |
|||||||||||||||
| Vapor pressure |
503.5 Torr (18.8 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Digermane is a chemical compound from the group of germane .
Extraction and presentation
Digerman can be obtained by passing Monogerman through a silent electrical discharge, with Digerman and Trigerman as main products and alongside higher Germans up to Nonagerman . Other processes for the production of Monogerman also produce higher Germans , such as the acid decomposition of magnesium germanide with ammonium bromide .
properties
Digerman is a colorless liquid that boils at 31 ° C. According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.95946, B = 1129.447 and C = −19.239 in the temperature range from 184.5 to 304.7 K. The enthalpy of vaporization at the boiling point is 26.8 kJ · mol −1 . The compound decomposes when heated at temperatures above 200 ° C.
use
Digerman is used for the deposition of amorphous germanium and epitaxial thin films . It is also used in the formation of boron- doped silicon- germanium alloy layers and with diborane in the formation of boron-doped germanium films using epitaxial processes.
Individual evidence
- ↑ a b c Dennis, LM; Corey, RB; Moore, RW: Germanium VII: The Hydrides of Germanium in J. Am. Chem. Soc. 46 (1924) 657-674, doi : 10.1021 / ja01668a015 .
- ↑ a b c d e data sheet Digerman, 9-11% (balance is hydrogen), 99.99% (excluding germane and trigermane), 10% in hydrogen, electronic grade at Sigma-Aldrich , accessed on January 16, 2014 ( PDF ).
- ↑ a b Emeleus, HJ; Gardner, ER: in J. Chem. Soc. 1938, 1900-1909.
- ↑ a b Georg Brauer (Ed.), With the collaboration of Marianne Baudler u a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 716.
- ^ Stull, DR: Vapor Pressure of Pure Substances. Organic and Inorganic Compounds in Ind. Eng. Chem. 39 (1947) 517-540, doi : 10.1021 / ie50448a022 .
- ↑ Emeleus, HJ; Jellinek, HHG: in Trans. Faraday Soc. 40 (1944) 93-99.

