Loline alkaloids
Loline alkaloids , also called loline for short , are bioactive natural substances that belong to the group of alkaloids . Loline alkaloids are produced in particular by the mushrooms from the ergot relatives family that live symbiotically on and in sweet grasses . They act as natural insecticides and protect both the fungus and the host plant from eating. Chemically, the loline alkaloids with their 1-amino-2,7-epoxypyrrolizidine structure belong to the pyrrolizidine alkaloids .
Occurrence
Loline alkaloids can be found in particular in sweet grasses, in particular in representatives of the genera Lolium , Festuca and Poa . But not the plant, but infection is responsible for the production of these alkaloids endophytic fungi of the genus Epichloë (and its as Neotyphodium designated anamorphic ) from the family of the ergot fungus responsible relatives.
Outside of the symbiotic community of sweet grass and endophyte, loline alkaloids only occur sporadically in nature. For example, an occurrence of loline alkaloids in Adenocarpus hispanicus (family: legumes ) and Argyreia mollis (family: bindweed ) has been reported.
biosynthesis
Despite their structural affiliation to the group of pyrrolizidine alkaloids, the biosynthesis of the loline alkaloids differs significantly from that of other pyrrolizidine alkaloids. In contrast to other pyrrolizidine alkaloids, loline alkaloids are not derived from ornithine or arginine , but are formed from the amino acids proline and homoserine . The primary biosynthetic product is 1-acetamidopyrrolizidine, which is cyclized to acetyltemulin. Other loline alkaloids are derived biosynthetically from this substance. Several are involved in the biosynthesis of loline alkaloid enzymes of LOL - gene cluster involved.
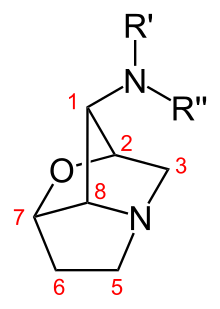
chemistry
structure
 |

|
| Temulin | Lolin |
Loline alkaloids are tricyclic pyrrolizidine alkaloids. The representatives of this class of substances differ in terms of the substituents on the amino group in position 1 of the loline skeleton. The structurally simplest loline alkaloid are Temulin with a free amino group and its N -methylierter descendant Lolin . The other loline alkaloids are derived from these substances and carry various acyl substituents .
synthesis
Various methods have been described for the synthesis of loline alkaloids.
The loline alkaloid synthesis described by Wilson starts from 8-oxabicyclo [3.2.1] oct-6-en-3-one, which is converted in several steps to 9-oxa-4-azabicyclo [4.2.1] non-7-ene . An intramolecular ring linkage in the presence of bromine leads to 2,7-epoxypyrrolizidine-1-bromide, which can be aminated to temulin or other loline alkaloids .
Starting from 1-ethylpyrrolidin-3-one after Cope elimination to give the corresponding nitrone , cycloaddition of ( E ) -methyl-4-hydroxy-2-butenoate leads to the intermediate ring closure to the hexahydropyrrolo [1,2- b ] [1,2] oxazole derivative, which is rearranged to the pyrrolizidine derivative after reduction of the NO bond in the sense of an S N 2 reaction . A Williamson ether synthesis enables the tetrahydrofuran ring to be closed . Lolin as the end product of the synthesis route is obtained by Curtius rearrangement with subsequent reduction starting from the intermediate product 2,7-epoxypyrrolizidine-1-carboxylic acid ethyl ether.
Another synthetic route starts from divinylcarbinol and 3-butenylamine and a ring closure to give the heyahydroazocine derivative. After azidation with lithium azide , in the presence of bromine, the transannular ring linkage to the pyrrolizidine derivative takes place in the sense of a combination of electrophilic and nucleophilic substitution mechanisms as a key step in the synthesis. The tetrahydrofuran ring is then closed by intramolecular etherification. The 2,7-epoxypyrrolizidine-1-azide obtained in this way can be reduced to temulin or methylated to lolin and reduced.
Biological importance
Loline alkaloids are, in addition to ergot alkaloids , lolitremes and peramin, a component of the feeding protection of the symbiotic community consisting of endophytes of the genus Epichloë / Neotyphodium and sweet grasses. Lolines have an insecticidal effect on a wide range of insects .
Individual evidence
- ^ Lane, Geoffrey A., Michael J. Christensen, and Christopher O. Miles: Coevolution of Fungal Endophytes with Grasses The Significance of Secondary Metabolites . In: Charles W. Bacon, James White (Eds.): Microbial Endophytes . CRC Press, 2000, ISBN 1420027115 , pp. 341-388.
- ↑ Tofern B, Kaloga M, Witte L, Hartmann T, Eich E: Occurrence of loline alkaloids in Argyreia mollis (Convolvulaceae) . In: Phytochemistry . 51, 1999, pp. 1177-1180. doi : 10.1016 / S0031-9422 (99) 00121-1 .
- Jump up ↑ Veen G, Greinwald R, Canto P, Witte L, Czygan FC: Alkaloids of Adenocarpus hispanicus (Lam.) DC varieties . In: Journal for Nature Research . 47, 1992, pp. 341-345.
- ↑ JD Blankenship, JB Houseknecht, S. Pal, LP Bush, RB Grossman, CL Schardl: Biosynthetic precursors of fungal pyrrolizidines, the loline alkaloids . In: ChemBioChem . 6, No. 6, 2005, pp. 1016-1022. doi : 10.1002 / cbic.200400327 . PMID 15861432 .
- ↑ CL Schardl, CA Young, U. Hesse, SG Amyotte, K. Andreeva, PJ Calie, DJ Fleetwood, DC Haws, N. Moore, B. Oeser, DG Panaccione, KK Schweri, CR Voisey, ML Farman, JW Jaromczyk, BA Roe, DM O'Sullivan, B. Scott, P. Tudzynski, Z. An, EG Arnaoudova, CT Bullock, ND Charlton, L. Chen, M. Cox, RD Dinkins, S. Florea, AE Glenn, A. Gordon , U. Güldener, DR Harris, W. Hollin, J. Jaromczyk, RD Johnson, AK Khan, E. Leistner, A. Leuchtmann, C. Li, J. Liu, J. Liu, M. Liu, W. Mace, C. Machado, P. Nagabhyru, J. Pan, J. Schmid, K. Sugawara, U. Steiner, JE Takach, E. Tanaka, JS Webb, EV Wilson, JL Wiseman, R. Yoshida, Z. Zeng: Plant- symbiotic fungi as chemical engineers: multi-genome analysis of the clavicipitaceae reveals dynamics of alkaloid loci . In: PLoS Genet . 9, No. 2, 2013, pp. E1003323 – e1003323. doi : 10.1371 / journal.pgen.1003323 . PMID 23468653 .
- ↑ a b C. L. Schardl, RB Grossman, P. Nagabhyru, JR Faulkner, UP Mallik: lolines alkaloids: Currencies of mutualism . In: Phytochemistry . 68, No. 7, 2007, pp. 980-996. doi : 10.1016 / j.phytochem.2007.01.010 . PMID 17346759 .
- ^ A b Wilson SR, Sawicki RA, Huffman JC: Synthetic and structural studies of the lolium alkaloids . In: J. Org. Chem. . 46, No. 19, 1981, pp. 3887-3891. doi : 10.1021 / jo00332a025 .
- ↑ a b Tufariello JJ, Meckler H, Winzenberg K: Synthesis of the lolium alkaloids . In: J. Org. Chem. . 51, No. 18, 1986, pp. 3556-3557. doi : 10.1021 / jo00368a035 .
- ↑ Blakemore PR, Kim SK, Schulze VK, White JD, Yokochia AF: Asymmetric synthesis of (+) - loline, a pyrrolizidine alkaloid from rye grass and tall fescue . In: J Chem Soc, Perkin Trans . 1, 2001, pp. 1831-1847. doi : 10.1039 / B103936A .
- ↑ a b Cakmak M, Mayer P, Trauner D: An efficient synthesis of loline alkaloids . In: Nat Chem . 3, No. 7, 2011, pp. 543-545. doi : 10.1038 / nchem.1072 . PMID 21697875 .