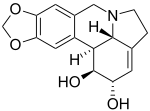
Lycorine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Lycorine | |||||||||||||||
| other names |
3,3α-Didehydrolycorane-1α, 2β-diol |
|||||||||||||||
| Molecular formula | C 16 H 17 NO 4 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 287.31 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
280-281 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Lycorin is an alkaloid that causes nausea, vomiting and diarrhea in low doses, and in higher doses it leads to cramps, paralysis and circulatory failure .
Occurrence
It is found in the Belladonnalilie , Hippeastrum hybrids and other representatives of the Amaryllis family , e.g. B. daffodils included.
Individual evidence
- ↑ a b Entry on Amaryllidaceae alkaloids. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.
- ↑ There is not yet a harmonized classification for this substance . A labeling of Lycorine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on July 7, 2020, is reproduced from a self-classification by the distributor .

