Lynestrenol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
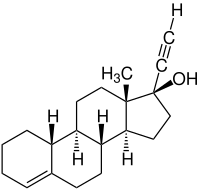
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Lynestrenol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 20 H 28 O | |||||||||||||||||||||
| Brief description |
white solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 284.44 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
158-160 ° C |
|||||||||||||||||||||
| solubility |
soluble in chloroform and ethanol |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Lynestrenol is a derivative ( derivative ) of the 19-Nortestosterons . It belongs to the group of synthetic gestagens and, through metabolism to norethisterone, develops a progesterone-like effect.
Chemically, lynestrenol belongs to the steroids .
Extraction and presentation
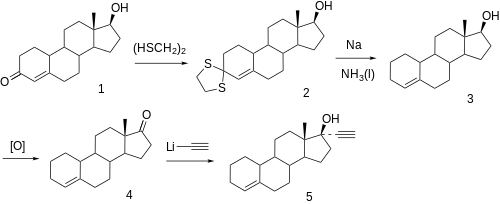
For the synthesis of lynestrenol nortestosterone is ( 1 ) by 1,2-ethanedithiol and boron trifluoride in the 3-position to the dithioketal ( 2 converted) and treated with sodium in ammonia to 17 β -hydroxy-estr-4-ene ( 3 ) is reduced, thereafter oxidized in position 17 with the Jones reagent to the ketone ( 4 ) and reacted with lithium acetylide to form lynestrenol ( 5 ).
properties
Physical Properties
It is a solid. The specific rotation value [α] 20 D is −9.5 ° to −11 ° (3.6% m / v in 96% ethanol ).
Chemical properties
Lynestrenol has as the natural steroid hormones a Steran -Grundgerüst. Lynestrenol is produced synthetically and differs from the natural steroid hormones in that it lacks a keto group on the C-3 atom of the sterile structure. The molecule is chiral and has six stereocenters.
History and use
Lynestrenol was introduced into medical therapy in 1969. It belongs to the first generation of synthetic gestagens and was used for contraception - mostly in combination with an estrogen ( birth control pill ) , but also alone ( minipill ). Today, more modern preparations of the 3rd and 4th generation are prescribed for contraception . Lynestrenol is also indicated for the treatment of menopausal symptoms ( postmenopausal hormone replacement treatment ). There are no approved drugs containing lynestrenol in the United States. Lynestrenol is still important for the history of the development of hormonal contraceptives, which have changed and improved over the years.
Metabolism
Lynestrenol is a prodrug and, after oral ingestion, is converted in the human body to norethisterone , which is pharmacologically active.
Finished medicinal products
Exluton (D, no longer available), Orgametril (A, NL)
literature
- H. Lüllmann, K. Mohr, M. Wehling, L. Hein: Pharmakologie und Toxikologie , Thieme Verlag 2016, 18th edition, 476–478. ISBN 978-3-13-368518-4 .
Individual evidence
- ↑ a b c d e Entry on Lynestrenol at TCI Europe, accessed on June 22, 2017.
- ^ A b The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; P. 976, ISBN 978-0-911910-00-1 .
- ↑ MS de Winter, CM Siegmann and SA Szpilfogel, Chem. Ind., 1959, 905 in: Houben-Weyl: Methods of Organic Chemistry Vol IV / 16 4th Edition :. Reduction I , 1980, 668, ISBN 978-3-13-200904-2 .
- ↑ European Pharmacopoeia, 6th edition, basic work, Deutscher Apotheker-Verlag Stuttgart, 2008.
- ^ H. Lüllmann, K. Mohr, A. Ziegler: Pocket Atlas of Pharmacology , Thieme Verlag 1990, 232-235, ISBN 3-13-707701-X .
- ↑ Annetine Gelijns (1991). Innovation in Clinical Practice: The Dynamics of Medical Technology Development. National Academies. P. 167–. NAP: 13513.
- ↑ Odlind V, Weiner E, Victor A, Johansson ED (1979). Plasma levels of norethindrone after single oral dose administration of norethindrone and lynestrenol. Clin. Endocrinol. (Oxf)., 1979, 10 (1): 29-38. PMID 436304 .