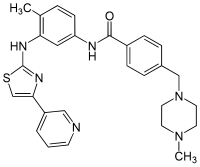
Masitinib
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Masitinib | |||||||||||||||
| other names |
4 - [(4-methylpiperazin-1-yl) methyl] - N - (4-methyl-3 - {[4- (pyridin-3-yl) -1,3-thiazol-2-yl] amino} phenyl) benzamide ( IUPAC ) |
|||||||||||||||
| Molecular formula | C 28 H 30 N 6 OS | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Mechanism of action |
Protein tyrosine kinase inhibitor |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 498.64 g · mol -1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Masitinib is a tyrosine kinase inhibitor that has been approved in veterinary medicine for the treatment of mast cell tumors in dogs . After approval in November 2008, masitinib has been available in Europe under the trade name Masivet since mid-2009. The veterinary medicinal product has been on the US market under the trade name Kinavet since 2011. Marketing authorization holder is AB Science from Paris. The drug is effective orally .
Mechanism of action
Masitinib selectively inhibits the mutated form of the c-kit tyrosine kinase in vitro . Such c-kit tyrosine kinase, which is permanently activated by mutations, plays a role in various forms of cancer. The selective action of masitinib does not affect other class III receptor tyrosine kinases .
Masitinib also inhibits the platelet growth factor receptor (PDGFR) and the fibroblast growth factor receptor (FGFR3).
Clinical development in human medicine
In human medicine, masitinib was or is being developed for the treatment of various cancers (gastrointestinal stromal tumors, acute myeloid leukemia , NSCLC , ovarian carcinoma , prostate carcinoma , multiple myeloma , metastatic pancreatic carcinoma , mast cell tumors, malignant melanoma in Alzheimer's disease ), Disease , and for the treatment of multiple sclerosis (as of 2016). Masitinib is also classified as an orphan drug for the treatment of pancreatic cancer in the EU .
Marketing authorization applications in the EU for masitinib for the treatment of gastrointestinal stromal tumors (planned trade name: Masican ) and pancreatic neoplasms (planned trade name: Masiviera ) were rejected in 2014.
EU approval for the treatment of systemic mastocytosis ( Masipro ) was refused in 2017. The CHMP questioned the reliability of the study results, as a review at the trial sites revealed "serious deficiencies" in the way the study was conducted. In addition, major changes to the study design were made while the study was ongoing and safety data are limited.
The EU approval of masitinib for the treatment of amyotrophic lateral sclerosis ( Alsitek ) was denied in 2018.
Chemical-pharmaceutical information
Masitinib is used medicinally as the salt of methanesulfonic acid (masitinib mesilate), which is very good in aqueous solutions at an acidic pH value, but is insoluble at an alkaline pH value.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Product information on Masivet on the website of the European Medicines Agency.
- ↑ I. Marech, R. Patruno, N. Zizzo, C. Gadaleta, M. Introna, AF Zito, CD Gadaleta, G. Ranieri: masitinib (AB1010), from canine tumor model to human clinical development: where we are? In: Critical reviews in oncology / hematology. Volume 91, Number 1, July 2014, pp. 98-111, doi: 10.1016 / j.critrevonc.2013.12.011 , PMID 24405856 .
- ↑ EU Clinical Trials Register; Retrieved August 30, 2011.
- ↑ J. Folch, D. Petrov, M. Ettcheto, S. Abad, E. Sánchez-López, ML García, J. Olloquequi, C. Beas-Zarate, C. Auladell, A. Camins: Current Research Therapeutic Strategies for Alzheimer's Disease Treatment. In: Neural plasticity. Volume 2016, 2016, p. 8501693, doi: 10.1155 / 2016/8501693 , PMID 26881137 , PMC 4735913 (free full text).
- ↑ A Phase 3 Study to Evaluate the Safety and Efficacy of Masitinib in Patients With Mild to Moderate Alzheimer's Disease .
- ↑ A. Shirani, DT Okuda, O. Stüve: Therapeutic Advances and Future Prospects in Progressive Forms of Multiple Sclerosis. In: Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. Volume 13, number 1, January 2016, pp. 58-69, doi: 10.1007 / s13311-015-0409-z , PMID 26729332 , PMC 4720678 (free full text).
- ↑ Orphan designation for masitinib on the website of the European Medicines Agency.
- ↑ a b Rejected human medicinal products in the EU community register , accessed on March 7, 2019.
- ^ Refusal of the marketing authorization for Masipro (masitinib) . (PDF) EMA, September 15, 2017; accessed on September 21, 2017
- ↑ D. Petrov, C. Mansfield, A. Moussy, O. Hermine: ALS Clinical Trials Review: 20 Years of Failure. Are We Any Closer to Registering a New Treatment? In: Frontiers in aging neuroscience. Volume 9, 2017, p. 68, doi: 10.3389 / fnagi.2017.00068 , PMID 28382000 , PMC 5360725 (free full text).