Mazindol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
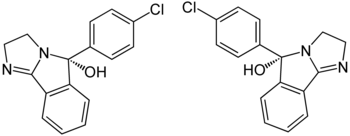
|
|||||||||||||||||||
| Mazindol enantiomers (1: 1 mixture) | |||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Mazindol | ||||||||||||||||||
| other names |
( RS ) -5- (4-chlorophenyl) -3,5-dihydro-2 H -imidazo [2,1- a ] isoindol-5-ol ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 16 H 13 ClN 2 O | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 284.74 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
202-203 ° C |
||||||||||||||||||
| solubility |
10 g l −1 in DMSO |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Mazindol is a tricyclic isoindole derivative and a drug from the group of psychostimulants . It is not related to the tricyclic antidepressants .
Mazindol has been out of trade in Germany since 1990.
Indications
Mazindol is used for the initial treatment of obesity . Therapeutically sensible doses are between 1 and 3 mg / day at breakfast. This therapy principle is now outdated. It was beyond his approval for the treatment of daytime sleepiness in narcolepsy used.
pharmacology
Pharmacodynamics
Mazindol has a similar effect to amphetamine but is not itself an amphetamine derivative. It inhibits v. a. the resumption of the messenger substance norepinephrine .
Pharmacokinetics
Mazindol develops its maximum effect (T max ) after 2 to 4 hours. The bioavailability is 50%. The half-life is 33 to 55 hours. It is excreted renally to 25% and biliary to 75% .
Side effects and interactions
- Common (1% -10%): Angina pectoris .
- Rare (<0.1%): pulmonary hypertension
- Also: insomnia, dizziness, agitation, confusion, mydriasis, somnolence, apathy, convulsions, coma; Difficulty urinating, urinary retention, impotence; Dry mouth, sweating, thirst, chills; Stomach pressure, constipation, diarrhea, ileus; rarely palpitations, rash, muscle twitching, tachycardia, hypotension, arrhythmias, shock.
literature
- Dykes, MHM: Evaluations of mazindol. In: Drug and Therap. Bull. 12 (1974), p. 1015.
- Heikkila, RE: Pharmacological studies with several analogs of mazindol: correlation between effects on dopamine uptake and various in vivo responses. In: Eur. J. Pharmacol. (71 (2-3) / 1981), pp. 277-86.
- Lean, ME: Ciclazindol: an oral agent with weight reducing properties and hypoglaecemic activity. In: Eur. J. Clin. Pharmacol. (25 (1) / 1983), pp. 41-45.
Individual evidence
- ↑ a b c d e Mazindol data sheet from Sigma-Aldrich , accessed on April 9, 2011 ( PDF ).
- ↑ Geert Mayer and a .: Narcolepsy: diagnosis and therapy . In: Deutsches Ärzteblatt , Heft 5, 2001, 98, pp. A249 – A254.