Metabotropic glutamate receptor 1
| Metabotropic glutamate receptor type 1 | ||
|---|---|---|

|
||
| Ribbon representation of the crystal structure 1EWK | ||
| other names |
mGluR1, mGlu1R |
|
|
Existing structure data : PDB 3KS9 , PDB 4OR2 , PDB 1ISR , PDB 1ISS , PDB 1EWK , PDB 1EWT |
||
| Properties of human protein | ||
| Mass / length primary structure | 1,194 amino acids , 132,357 Da | |
| Isoforms | 1a, 1b, 1c, 1d, 1e | |
| Identifier | ||
| External IDs | ||
| Orthologue | ||
| human | House mouse | |
| Entrez | 2911 | 14816 |
| Ensemble | ENSG00000152822 | ENSMUSG00000019828 |
| UniProt | Q13255 | P97772 |
| Refseq (mRNA) | NM_001278064.1 | NM_001114333.2 |
| Refseq (protein) | NP_001264993.1 | NP_001107805.1 |
| Gene locus | ||
| PubMed search | 2911 |
14816
|
Metabotropic glutamate receptor 1 (GRM 1, mGluR1) is a protein from the group of metabotropic glutamate receptors .
properties
The excitatory neurotransmitter glutamate binds to the orthosteric agonist binding site of the receptor (the binding site of the natural ligand glutamate) and induces or stabilizes a conformation of the protein tertiary structure , which enables the coupling of G proteins (G q , G s and G i / o ) and thus causes signal transduction . As a result, the secondary messenger substances IP 3 and diacylglycerol (DAG) are formed and calcium ions are released from the endoplasmic reticulum into the cytosol . DAG activates protein kinase C . The metabotropic glutamate receptor 1 is involved in the effect of glutamate in the brain , including long-term potentiation in the hippocampus and long-term depression in the cerebellum .
mGluR1 is found at many synapses in the central nervous system ; its expression is highest at the parallel fiber synapse of the Purkinje cells in the cerebellum. The C -terminal end of the protein is responsible for its location. In this region of the protein structure there are domains that bind the receptor in the dendrite . These domains occur redundantly in the structure of mGluR1. For its postsynaptic localization, however, z. B. is responsible for the binding site for the postsynaptic protein Homer .
The metabotropic glutamate receptor 1, like the metabotropic glutamate receptor 4 , is probably involved in the umami taste on the tongue.
structure
The metabotropic glutamate receptor type 1 is a class C G protein-coupled receptor . It was first described as the first member of its receptor family in 1991. Together with the metabotropic glutamate receptor 5 , it forms group I of the metabotropic glutamate receptors . Its three-dimensional structure was first published in 2000. Since then, other (crystal) structures have become known which reflect different conformational functional states through complexation with various types of ligands .
Like other metabotropic receptors, mGluR1 is a membrane protein . The isoforms 1a-1d each have seven α-helical transmembrane - domains ; the shortest isoform 1e, however, is none.
The receptor forms dimers , which are mostly secreted as constitutive functional units from the endoplasmic reticulum to the cell membrane . A dimerization option is offered by a cysteine located in the extracellular domain , which can form a disulfide bridge and thus covalent adducts with other suitable proteins . Examples are homodimers of identical receptors, homodimers of splice variants , family- specific heterodimers with the metabotropic glutamate receptor 5, but not with glutamate receptors of groups II and III. Non-family heteromerization is possible with other class C receptors (C-GPCR), such as the calcium-sensitive receptor . The dimerization possibilities are neither limited to the disulfide bridges nor to a single binding site. The receptor mGluR1- Adenosin1R is alien to the class . The metabotropic glutamate receptor type 1 has disulfide bridges and is glycosylated and phosphorylated .
Ligands
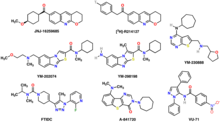
Quisqualate , dihydroxyphenylglycine (DHPG) and 1-amino-1,3-dicarboxycyclopentane (ACPD) are functionally selective mGlur1 agonists . An inhibitor of the metabotropic glutamate receptor 1 is LY341495 , the binding site of which partially overlaps with that of glutamate. An allosteric inhibitor is FITM ( 4-fluoro-N- (4- (6- (isopropylamino) pyrimidin-4-yl) thiazol-2-yl) -N-methylbenzamide ), which binds in a binding pocket near the transmembrane helix.
In addition to the binding site of the natural ligand, there are two allosteric binding sites of the mGluR1.
Further ligands of the mGluR1 are:
- JNJ-16259685: high affinity selective non-competitive antagonist
- R-214,127: high affinity selective allosteric antagonist
- YM-202,074: high affinity selective allosteric antagonist
- YM-230,888: high affinity selective allosteric antagonist
- YM-298,198: selective non-competitive antagonist
- FTIDC: high affinity selective allosteric antagonist
- A-841,720: selective non-competitive antagonist; slight binding to hmGluR5
- VU-71: amplifier
- Fluorinated 9 H -xanthene-9-carboxylic acid-oxazol-2-yl-amides: orally available enhancers
- Cyclothiazide : non-selective non-competitive antagonist
literature
- Katharine Herrick-Davis, Graeme Milligan, Giuseppe Di Giovanni: G-Protein-Coupled Receptor Dimers. Springer, 2017, ISBN 978-3-319-60174-8 , pp. 327-344.
Web links
Individual evidence
- ↑ a b c d e f g GRM1 - Metabotropic glutamate receptor 1 precursor - Homo sapiens (Human) - GRM1 gene & protein. In: uniprot.org. September 12, 2018, accessed October 8, 2018 .
- ↑ K. Yasumatsu, T. Manabe, R. Yoshida, K. Iwatsuki, H. Uneyama, I. Takahashi, Y. Ninomiya: Involvement of multiple taste receptors in umami taste: analysis of gustatory nerve responses in metabotropic glutamate receptor 4 knockout mice . In: Journal of Physiology. Volume 593, No. 4, February 2015, pp. 1021-1034; doi: 10.1113 / jphysiol.2014.284703 , PMC 4398535 (free full text).
- ↑ X. Li et al.: Human receptors for sweet and umami taste. In: Proc. Natl. Acad. Sci. USA 99 (7), 2002, pp. 4692-4696. PMID 11917125 . (PDF) .
- ↑ Houamed KM, Kuijper JL, Gilbert TL, et al: Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain . In: Science . 252, No. 5010, May 1991, pp. 1318-21. PMID 1656524 .
- ↑ Gama L, Wilt SG, Breitwieser GE: Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons . In: J. Biol. Chem. . 276, No. 42, October 2001, pp. 39053-9. doi : 10.1074 / jbc.M105662200 . PMID 11489900 .
- ↑ Kamikubo Y, Tabata T, Sakairi H, Hashimoto Y, Sakurai T: Complex formation and functional interaction between adenosine A1 receptor and type-1 metabotropic glutamate receptor . In: J. Pharmacol. Sci. . 128, No. 3, July 2015, pp. 125-30. doi : 10.1016 / j.jphs.2015.06.002 . PMID 26154847 .
- ↑ K. Wisniewski, H. Car: (S) -3,5-DHPG: a review. In: CNS Drug Reviews Volume 8, Number 1, 2002, pp. 101-116, ISSN 1080-563X . PMID 12070529 .
- ↑ Hathaway HA, Pshenichkin S, Grajkowska E, et al: Pharmacological characterization of mGlu1 receptors in cerebellar granule cells reveals biased agonism . In: Neuropharmacology . 93, June 2015, pp. 199–208. doi : 10.1016 / j.neuropharm.2015.02.007 . PMID 25700650 . PMC 4387075 (free full text).
- ↑ Emery AC, DiRaddo JO, Miller E, etal: Ligand bias at metabotropic glutamate 1a receptors: molecular determinants that distinguish β-arrestin-mediated from G protein-mediated signaling . In: Mol. Pharmacol. . 82, No. 2, August 2012, pp. 291-301. doi : 10.1124 / mol.112.078444 . PMID 22584219 . PMC 3400838 (free full text).
- ↑ a b Hemstapat K, de Paulis T, Chen Y, Brady AE, Grover VK, Alagille D, Tamagnan GD, Conn PJ: A novel class of positive allosteric modulators of metabotropic glutamate receptor subtype 1 interact with a site distinct from that of negative allosteric modulators . In: Mol. Pharmacol. . 70, No. 2, 2006, pp. 616-26. doi : 10.1124 / mol.105.021857 . PMID 16645124 .
- ↑ Lavreysen H, Wouters R, Bischoff F, Nóbrega Pereira S, Langlois X, Blokland S, Somers M, Dillen L, Lesage AS: JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist . In: Neuropharmacology . 47, No. 7, 2004, pp. 961-72. doi : 10.1016 / j.neuropharm.2004.08.007 . PMID 15555631 .
- ↑ Lavreysen H, Janssen C, Bischoff F, Langlois X, Leysen JE, Lesage AS: [3H R214127: a novel high-affinity radioligand for the mGlu1 receptor reveals a common binding site shared by multiple allosteric antagonists.] In: Mol. Pharmacol . . 63, No. 5, 2003, pp. 1082-93. doi : 10.1124 / mol.63.5.1082 . PMID 12695537 .
- ↑ Kohara A, Takahashi M, Yatsugi S, Tamura S, Shitaka Y, Hayashibe S, Kawabata S, Okada M: Neuroprotective effects of the selective type 1 metabotropic glutamate receptor antagonist YM-202074 in rat stroke models . In: Brain Res. . 1191, 2008, pp. 168-79. doi : 10.1016 / j.brainres.2007.11.035 . PMID 18164695 .
- ↑ Kohara A, Nagakura Y, Kiso T, Toya T, Watabiki T, Tamura S, Shitaka Y, Itahana H, Okada M: Antinociceptive profile of a selective metabotropic glutamate receptor 1 antagonist YM-230888 in chronic pain rodent models . In: Eur. J. Pharmacol. . 571, No. 1, 2007, pp. 8-16. doi : 10.1016 / j.ejphar.2007.05.030 . PMID 17597604 .
- ↑ Kohara A, Toya T, Tamura S, Watabiki T, Nagakura Y, Shitaka Y, Hayashibe S, Kawabata S, Okada M: Radioligand binding properties and pharmacological characterization of 6-amino-N-cyclohexyl-N, 3-dimethylthiazolo [3 , 2-a] benzimidazole-2-carboxamide (YM-298198), a high-affinity, selective, and noncompetitive antagonist of metabotropic glutamate receptor type 1 . In: J. Pharmacol. Exp. Ther. . 315, No. 1, 2005, pp. 163-9. doi : 10.1124 / jpet.105.087171 . PMID 15976016 .
- ↑ Suzuki G, Kimura T, Satow A, Kaneko N, Fukuda J, Hikichi H, Sakai N, Maehara S, Kawagoe-Takaki H, Hata M, Azuma T, Ito S, Kawamoto H, Ohta H: Pharmacological characterization of a new , orally active and potent allosteric metabotropic glutamate receptor 1 antagonist, 4- [1- (2-fluoropyridin-3-yl) -5-methyl-1H-1,2,3-triazol-4-yl] -N-isopropyl- N-methyl-3,6-dihydropyridine-1 (2H) -carboxamide (FTIDC) . In: J. Pharmacol. Exp. Ther. . 321, No. 3, 2007, pp. 1144-53. doi : 10.1124 / jpet.106.116574 . PMID 17360958 .
- ↑ El-Kouhen O, Lehto SG, Pan JB, Chang R, Baker SJ, Zhong C, Hollingsworth PR, Mikusa JP, Cronin EA, Chu KL, McGaraughty SP, Uchic ME, Miller LN, Rodell NM, Patel M, Bhatia P. , Mezler M, Kolasa T, Zheng GZ, Fox GB, Stewart AO, Decker MW, Moreland RB, Brioni JD, Honore P: Blockade of mGluR1 receptor results in analgesia and disruption of motor and cognitive performances: effects of A-841720, a novel non-competitive mGluR1 receptor antagonist . In: Br. J. Pharmacol. . 149, No. 6, 2006, pp. 761-74. doi : 10.1038 / sj.bjp.0706877 . PMID 17016515 . PMC 2014656 (free full text).
- ↑ Vieira E, Huwyler J, Jolidon S, Knoflach F, Mutel V, Wichmann J: Fluorinated 9H-xanthene-9-carboxylic acid oxazol-2-yl-amides as potent, orally available mGlu1 receptor enhancers . In: Bioorg. Med. Chem. Lett. . 19, No. 6, 2009, pp. 1666-9. doi : 10.1016 / j.bmcl.2009.01.108 . PMID 19233648 .
- ↑ Surin A, Pshenichkin S, Grajkowska E, Surina E, Wroblewski JT: Cyclothiazide selectively inhibits mGluR1 receptors interacting with a common allosteric site for non-competitive antagonists . In: Neuropharmacology . 52, No. 3, 2007, pp. 744-54. doi : 10.1016 / j.neuropharm.2006.09.018 . PMID 17095021 . PMC 1876747 (free full text).