Michler's base
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
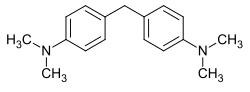
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Michler's base | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 17 H 22 N 2 | ||||||||||||||||||
| Brief description |
white to bluish white solid with a characteristic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 254.38 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.039 g cm −3 |
||||||||||||||||||
| Melting point |
90-91 ° C |
||||||||||||||||||
| boiling point |
390 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Authorization procedure under REACH |
particularly worrying : carcinogenic ( CMR ) |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Michler's base is a chemical compound from the group of aminobenzenes and diarylmethanes . It is named after the German chemist Wilhelm Michler (1846–1889).
Extraction and presentation
Michler's base can be obtained by reacting dimethylaniline with 40% formaldehyde and concentrated hydrochloric acid , by reacting dimethylaniline with diacetyl peroxide or by reacting dimethylaniline with tert-butyl perbenzoate .
properties
Michler's base is a flammable, hardly inflammable, white to bluish-white solid with a characteristic odor, which is practically insoluble in water. It decomposes when heated.
use
Michler's base is used as an intermediate in the production of dyes and serves as a reagent for the determination of lead.
Individual evidence
- ↑ a b c d e f g h Entry on N, N, N ′, N′-tetramethyl-4,4′-methylenedianiline in the GESTIS substance database of the IFA , accessed on July 8, 2019(JavaScript required) .
- ↑ a b ECHA: SUPPORT DOCUMENT FOR IDENTIFICATION OF N, N, N ', N'-tetramethyl-4,4'-methylenedianiline (Michler's base) PROPOSAL FOR IDENTIFICATION OF A SUBSTANCE AS A CMR 1A OR 1B, PBT, vPvB OR A SUBSTANCE OF AN EQUIVALENT LEVEL OF CONCERN , accessed July 8, 2019.
- ↑ Data sheet 4,4′-Methylenebis (N, N-dimethylaniline), 98 +% from AlfaAesar, accessed on July 8, 2019 ( PDF )(JavaScript required) .
- ↑ Entry on N, N, N ′, N′-tetramethyl-4,4′-methylenedianiline in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 8, 2019. Manufacturers or distributors can use the harmonized classification and labeling expand .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on April 21, 2020.
- ↑ Entry on BIS (P- (DIMETHYLAMINO) PHENYL) METHANES in the Hazardous Substances Data Bank , accessed on July 8, 2019.
- ↑ Data sheet 4,4′-Methylenebis (N, N-dimethylaniline), 98% from Sigma-Aldrich , accessed on July 8, 2019 ( PDF ).

