tert -butyl peroxybenzoate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Wedges to clarify the geometry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | tert -butyl peroxybenzoate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 11 H 14 O 3 | |||||||||||||||
| Brief description |
clear, light yellow or yellowish liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 194.23 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
76-79 ° C at 0.2 mmHg |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
tert -Butyl peroxybenzoate (TBPB) is a perester of the general structure R 1 -C (O) OO-R 2 with a phenyl group as R 1 and a tert-butyl group as R 2 , which is often used as a free radical initiator for the polymerization of z. B. ethylene to LDPE and for the crosslinking of z. B. unsaturated polyester resins is used. The perester falls within the scope of the Explosives Act and is divided into substance group C in Appendix II.
Occurrence and representation
A standard method for the preparation of peresters is the acylation of tert -butyl hydroperoxide with benzoyl chloride ,
a large excess of tert-butyl hydroperoxide is used, the hydrogen chloride formed is removed in vacuo and a practically quantitative yield is thus achieved.
properties
tert -Butyl peroxybenzoate is a clear, light yellow liquid that is little in water, in many organic solvents, such as. B. ethanol or phthalate is soluble.
As a peroxo compound TBPB contains about 8.16 weight percent active oxygen and has a so-called self-accelerating decomposition temperature (SADT, self accelerating decomposition temperature ) of about 60 ° C. The SADT is the lowest temperature at which self-accelerating decomposition can occur in the transport packaging within a week and which must not be exceeded during storage and transport.
TBPB should therefore be stored between a minimum of 10 ° C (including solidification) and a maximum of 50 ° C. Dilution with a high boiling solvent increases the SADT. The half-life of TBPB at which 50% of the peroxyester is decomposed is 10 hours at 104 ° C, one hour at 124 ° C, and one minute at 165 ° C. Amines, metal ions, strong acids and bases, as well as strong reducing and oxidizing agents accelerate the decomposition of TBPB even in low concentrations.
Nonetheless, tert-butyl peroxybenzoate is one of the safest peresters or organic peroxides to handle. The main decomposition products of TBPB are carbon dioxide , acetone , methane , tert-butanol , benzoic acid and benzene .
Applications
The protective group 2-trimethylsilylethanesulfonylchloride (SES-Cl) for primary and secondary amino groups is accessible by reacting vinyltrimethylsilane with sodium hydrogen sulfite and tert -butylperoxybenzoate to the sodium salt of trimethylsilylethanesulfonic acid and subsequent reaction with thionyl chloride to form the corresponding sulfonyl chloride .
tert -Butyl peroxybenzoate is used to introduce the benzoyloxy group in the allyl position of unsaturated hydrocarbons .
From cyclohexene , with TBPB in the presence of catalytic amounts of copper (I) bromide, 3-benzoyloxycyclohexene is produced in 71 to 80% yield.
This allylic oxidation of alkenes, also known as the Kharasch -Sosnovsky oxidation
generates racemic allylic benzoates in the presence of catalytic amounts of copper (I) bromide.
A modification of the reaction uses copper (II) trifluoromethanesulfonate as a catalyst - as well as DBN or DBU as bases - and achieves yields of up to 80% in the conversion of acyclic olefins with TBPB to form allylic benzoates.
Substituted oxazolines and thiazolines can be oxidized in useful yields to the corresponding oxazoles and thiazoles in a modified Kharasch-Sosnovsky oxidation with tert-butyl peroxybenzoate and a mixture of Cu (I) and Cu (II) salts .
The carboalkoxy group at the C-4 position is essential for the reaction to succeed.
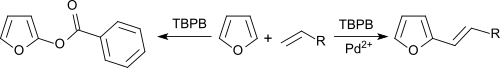
Benzene and furans can be alkylated with olefins in an oxidative coupling with palladium salt catalysis , with tert-butyl peroxybenzoate acting as a hydrogen acceptor.
In the absence of Pd 2+ salts, benzoxylation of the aromatics takes place.
The main application of tert -butyl peroxybenzoate is as a radical initiator, which allows the polymerization of monomers to form polymers , such as. B. from ethylene to LDPE, vinyl chloride to PVC , styrene to polystyrene or acrylic acid esters to polyacrylates initiated and as so-called heat hardeners for unsaturated polyester resins (English. Unsaturated polyester resins , UP resins). The quantities used for curing UP resins are approx. 1–2%.
A disadvantage, especially in the production of polymers for applications in the food or cosmetics sector, is the possible formation of benzene as a decomposition product, which can diffuse out of the polymer (e.g. an LDPE packaging film).
Risk assessment
In 2013, tert-butyl peroxybenzoate was included by the EU in the Community's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of tert-butyl peroxybenzoate were concerns about consumer and widespread use as well as the suspected dangers of sensitizing properties. The re-evaluation took place from 2013 and was carried out by Italy .
Individual evidence
- ↑ a b c d e f g data sheet tert-butyl peroxybenzoate from Sigma-Aldrich , accessed on May 20, 2016 ( PDF ).
- ↑ a b c d e f g h i Entry on tert-butyl perbenzoate in the GESTIS substance database of the IFA , accessed on May 20, 2016(JavaScript required) .
- ↑ Arkema: GPS Safety Summary, Substance Name: Tert-Butyl peroxybenzoate .
- ↑ a b Data sheet tert-Butyl peroxybenzoate, 98% from AlfaAesar, accessed on May 20, 2016 ( PDF )(JavaScript required) .
- ^ Sprengstoffgesetz - SprengG , accessed on November 6, 2018.
- ^ NA Milas, DG Orphanos, RJ Klein: The solvolysis of acid chlorides with t-alkyl hydroperoxides . In: J. Org. Chem. Band 29 , no. 10 , 1964, pp. 3099-3100 , doi : 10.1021 / jo01033a525 .
- ↑ a b c United Initiators, Technical Data Sheet, TBPB .
- ↑ a b Organic Peroxide Producers Safety Division, SAFETY AND HANDLING OF ORGANIC PEROXIDES The Society of the Plastics Industry, 2012 edition ( Memento of the original from April 1, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ a b S.M. Weinreb, CE Chase, P. Wipf, S. Venkatraman: 2-Trimethylsilylethanesulfonyl chloride (SES-Cl) In: Organic Syntheses . 75, 1998, p. 161, doi : 10.15227 / orgsyn.075.0161 ( PDF ).
- ↑ PERGAN GmbH: Organic peroxides for polymerisation .
- ↑ K. Pedersen, P. Jakobsen, S.-O. Lawesson: 3-Benzoyloxycyclohexene In: Organic Syntheses . 48, 1968, p. 18, doi : 10.15227 / orgsyn.048.0018 ; Coll. Vol. 5, 1973, p. 70 ( PDF ).
- ↑ MS Kharasch, G. Sosnovsky: The reactions of t-butyl perbenzoate and olefins - a stereospecific reaction . In: J. Amer. Chem. Soc. tape 80 , no. 3 , 1958, pp. 756-756 , doi : 10.1021 / ja01536a062 .
- ↑ G. Sakar, A. Datta Gupta, VK Singh: Cu (OTf) 2 - DBN / DBU complex as an efficient catalyst for allylic oxidation of olefins with tert-butyl perbenzoate . In: Tetrahedron Lett. tape 37 , no. 46 , 1996, pp. 8435-8436 , doi : 10.1016 / 0040-4039 (96) 01911-9 .
- ^ AI Meyers, FX Tavares: Oxidation of Oxazolines and Thiazolines to Oxazoles and Thiazoles. Application of the Kharasch - Sosnovsky Reaction . In: J. Org. Chem. Band 61 , no. 23 , 1996, pp. 8207-8215 , doi : 10.1021 / jo.9613491 .
- ↑ J. Tsuji, H. Nagashima: Palladium-catalyzed oxidative coupling of aromatic compounds with olefins using t-butyl perbenzoate as a hydrogen accepter . In: Tetrahedron . tape 40 , no. 14 , 1984, pp. 2699-2702 , doi : 10.1016 / S0040-4020 (01) 96888-7 .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): tert-butyl perbenzoate , accessed on May 20, 2019.