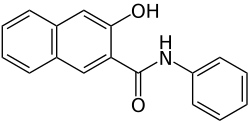
Naphthol AS
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Naphthol AS | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 17 H 13 NO 2 | |||||||||||||||
| Brief description |
white to reddish yellow solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 263.29 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Naphthol AS (also naphthol AS ) is a group of Naphthol - derivatives , as coupling component (CI Coupling Component) for development dyes are used.
Manufacture and properties
This class of compounds of coupling components can be produced by reacting 2-hydroxynaphthalene-3-carboxylic acid with phosphorus trichloride and variously substituted anilines (e.g. toluidines , xylidines , anisidines ). The name “Naphthol AS” (AS for “amide of acid”) was chosen for the parent compound, substituted derivatives were identified with the following letters (e.g. Naphthol AS-MX, Naphthol AS-TR).
Naphthol-AS derivatives form mono- and disodium salts with caustic soda , which are very strongly adsorbed by cellulose fibers.
Dyeing process
During development dyeing, cotton textiles are pretreated with naphthol AS derivatives and then mixed with diazonium salts of aromatic amines (“real bases”, “real salts”, e.g. fast red TR base ). The azo coupling of these salts to the adsorbed coupling component results in particularly wash-resistant dyeings or dyes. Thanks to the large selection of different naphthol AS derivatives and coloring salts, almost any color can be achieved.
Applications
Naphthol AS is used to dye textiles . The derivatives of naphthol-AS-phosphates (e.g. naphthol-AS-MX-phosphate ) are used for immunostaining with an alkaline phosphatase as a reporter enzyme.
history
The chemists LA Laska , Arthur Zitscher and Adolf Winther found in 1911 in the factory of the K. Oehler Anilin- und Anilinfarbenfabrik Offenbach that the anilide of 2-hydroxynaphthalene-3-carboxylic acid is particularly well suited as a coupling component to light and to produce washable azo dyes . This type of coupling component was protected by the Griesheim-Elektron company in Offenbach under the trade name Naphtol AS . The plant was later renamed Naphtol-Chemie Offenbach .
literature
- W. Kirst, W. Neumann: On the history of the development of Naphtol AS dyes . In: Angewandte Chemie . tape 66 , no. August 15 , 1954, p. 429-434 , doi : 10.1002 / anie.19540661502 .
Individual evidence
- ↑ a b c d Entry on 3-Hydroxy-2-naphthanilide at TCI Europe, accessed on June 2, 2016.
- ↑ a b Willy Herbst, Klaus Hunger: Industrial organic pigments production, properties, application , 3rd edition, Wiley-VCH, 2009, ISBN 3-527-62496-1 , p. 201.
- ^ Paul Heermann: Technology of textile finishing . Springer-Verlag, 1921, ISBN 978-3-642-99410-4 , p. 405 ( limited preview in Google Book search).
- ↑ Patent DE256999 : Process for the representation of monoazo dyes particularly suitable for pigment color preparation. Registered July 4, 1911 , published February 22, 1913 .
- ↑ Patent DE261594 : Process for the preparation of monoazo dyes. Registered May 18, 1912 , published June 27, 1913 .
- ↑ Brand name Naphtol AS, Reg.Nr. 345,595, August 31, 1925 subsequent owner logged Hoechst and DyStar , Brand deleted 31 August 2015 - the spelling in the Anglo-Saxon is / was often "naphth h modified ol AS".

