Native state

In biochemistry, the native state (synonymous native conformation ) is a conformation of biopolymers with the lowest free energy . The removal of the native state is called denaturation .
properties
Some biopolymers such as proteins and nucleic acids have a preferred lowest energy conformation. According to the principle of the least constraint, the most favorable conformation depends on the respective environmental conditions. For non-secreted biopolymers, the native state exists under the physiological conditions within a cell . The native state of a purified biopolymer can be determined using NMR spectroscopy under native conditions.
Proteins
In the case of proteins, the native state is the protein fold , which develops independently (partly with the help of chaperones ). In metastable proteins, the native folding is not necessarily the lowest free energy conformation. Correct folding is necessary for the protein to perform its functions, e.g. B. protein-protein interactions , protein-DNA interactions , protein-RNA interactions or protein-lipid interactions and, in the case of enzymes, additionally for the enzyme activity of the catalytic center . Incorrectly folded proteins are recognized by the protein quality control. The energies of different protein folds are represented as folding funnels based on the Anfinsen dogma . The Levinthal paradox provides an indication that not all possible conformations are tried out when folding a protein, but that a cumulative selection of individual, partially parallel folding steps takes place. The typical angles when two amino acids are rotated against each other ( dihedral angles ) are shown in a Ramachandran or Janin plot . In the case of proteins with a molten globule shape, metamorphic proteins or in the extreme case of intrinsically unstructured proteins , the dihedral angles are not fixed, but rather have a range of permitted degrees and thus permitted conformations.
Nucleic acids
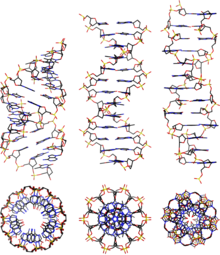
Two mutually complementary strands of nucleic acids such as DNA or RNA form one of the double helix structures due to the base pairing and stacking , depending on the environmental conditions in the form of A-DNA , B-DNA or Z-DNA . The main form present in cells is B-DNA.
carbohydrates
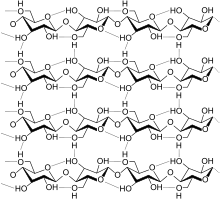
Some polysaccharides also have a preferred structure. Cellulose is insoluble in water in its native state. Cellulose is denatured during the production of viscose or cupro and temporarily soluble in water. After the fiber has been produced, it is returned to insolubility by a reversible reaction (viscose) or dilution of the denaturant (cupro).
Individual evidence
- ^ S. Govindarajan, RA Goldstein: On the thermodynamic hypothesis of protein folding. In: Proceedings of the National Academy of Sciences . Volume 95, Number 10, May 1998, pp. 5545-5549, PMID 9576919 , PMC 20414 (free full text).
- ↑ Jeremy M. Berg: Stryer Biochemistry. Springer-Verlag, 2017, ISBN 978-3-662-54620-8 , p. 64.
- ↑ Tanja Wüstenberg: Cellulose and cellulose derivatives. Behr's Verlag DE, 2013, ISBN 978-3-954-68188-4 , p. 15.