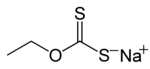
Sodium O- ethyl dithiocarbonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium O- ethyl dithiocarbonate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 5 NaOS 2 | |||||||||||||||
| Brief description |
light yellow solid with an unpleasant, carbon disulfide-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 144.18 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.263 g cm −3 |
|||||||||||||||
| Melting point |
69-69.5 ° C |
|||||||||||||||
| solubility |
easily soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium O- ethyldithiocarbonate is a chemical compound from the group of organosulfur compounds .
Extraction and presentation
Sodium O -ethyldithiocarbonat can as most xanthates by the treatment of sodium ethoxide with carbon disulfide are produced.
properties
Sodium O- ethyldithiocarbonate is a light yellow solid with an unpleasant, carbon disulfide-like odor, which is easily soluble in water in water. It decomposes when heated.
use
Sodium O- ethyldithiocarbonate is used in the extraction of copper and other metal ores by flotation .
Individual evidence
- ↑ a b c d e f Entry on sodium O-ethyldithiocarbonate in the GESTIS substance database of the IFA , accessed on December 9, 2018(JavaScript required) .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ G. Ingram, BA Toms: 23. The reactions of sodium ethyl xanthate with ethanol and with water. In: Journal of the Chemical Society. 1961, p. 117, doi : 10.1039 / JR9610000117 .
- ↑ Meso Florence Mokgethwa, Abraham Adewale Adeleke u. a .: An Evaluation of Sodium Ethyl Xanthate for the Froth Flotation Upgrading of a Carbonatitic Copper Ore. In: Journal of Physical Science. 27, 2016, p. 13, doi : 10.21315 / jps2016.27.2.2 .
- ↑ Frank Crundwell, Michael Moats, Venkoba Ramachandran: Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals . Elsevier, 2011, ISBN 978-0-08-096809-4 , pp. 178 ( limited preview in Google Book search).