Nickel (II) selenate
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
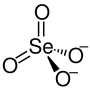
|
|||||||||||||
| General | |||||||||||||
| Surname | Nickel (II) selenate | ||||||||||||
| Molecular formula | NiSeO 4 | ||||||||||||
| Brief description |
green solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 201.64 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| density |
|
||||||||||||
| solubility |
soluble in water (350 g l −1 at 20 ° C) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Nickel (II) selenate is an inorganic chemical compound of nickel from the selenate group .
Occurrence
Nickel selenate occurs naturally in the form of a mixed crystal with cobalt selenate as the mineral ahlfeldite .
Extraction and presentation
Nickel selenate can be obtained by reacting nickel (II) carbonate with selenic acid .
properties
As a hexahydrate, nickel selenate is a green solid. It has a tetragonal crystal structure with the space group P 4 1 2 1 2 (space group no. 92) . The tetrahydrate, which slowly develops when the hexahydrate is heated from about 100 ° C, has a crystal structure with the space group P 2 1 / n (space group no. 14, position 2) , the dihydrate has an orthorhombic crystal structure. From 510 ° C the compound decomposes, with nickel selenite as well as nickel (II) oxide and selenium dioxide being formed with the elimination of oxygen .
Individual evidence
- ↑ a b c d Entry on nickel selenate in the GESTIS substance database of the IFA , accessed on December 30, 2019(JavaScript required) .
- ↑ a b R. J. Meyer: Nickel Part B - Delivery 2. Connections to Nickel-Polonium . Springer-Verlag, 2013, ISBN 978-3-662-13302-6 ( limited preview in Google book search).
- ^ A b William M. Haynes: CRC Handbook of Chemistry and Physics, 94th Edition . CRC Press, 2016, ISBN 978-1-4665-7115-0 , pp. 78 ( limited preview in Google Book search).
- ↑ Entry on nickel selenate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 30, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Jane E. Macintyre: Dictionary of Inorganic Compounds, Supplement 4 . CRC Press, 1996, ISBN 978-0-412-75020-5 , pp. 219 ( limited preview in Google Book search).
- ↑ H. Fuess: The crystal structure of nickel selenate hexahydrate. In: Journal of Inorganic and General Chemistry. 379, 1970, p. 204, doi : 10.1002 / zaac.19703790212 .
- ↑ D. Stoilova, V. Koleva: TG, DTA, DSC and X-ray powder diffraction studies on some nickel selenate hydrates. In: Thermochimica Acta. 290, 1997, p. 85, doi : 10.1016 / S0040-6031 (96) 03062-6 .



![{\ displaystyle \ mathrm {NiSeO_ {4} \ cdot 6H_ {2} O \ {\ xrightarrow [{- H_ {2} O}] {300 \, ^ {\ circ} C}} \ NiSeO_ {4} \ cdot H_ {2} O \ {\ xrightarrow [{- H_ {2} O}] {390 \, ^ {\ circ} C}} \ NiSeO_ {4} \ {\ xrightarrow [{- O_ {2}}] { 510 \, ^ {\ circ} C}} \ NiSeO_ {3} \ {\ xrightarrow [{- SeO_ {2}}] {690 \, ^ {\ circ} C}} \ NiO}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ae9bca30b2eaa6cc17535421ace34846e6081d83)