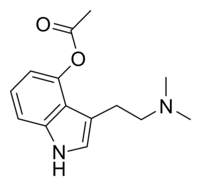
O -acetylpsilocin
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | O -acetylpsilocin | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 14 H 18 N 2 O 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 246.30 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
170–176 ° C (fumarate, decomposition) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
O -acetylpsilocin (also known as psilacetin , 4-acetoxy-DMT, or 4-AcO-DMT ) is a synthetically produced psychotropic drug and wassuggestedby David Nichols as a potentially useful alternative to psilocybin for pharmacological studies. Both are believed to be prodrugs of psilocin . However, some users report that the subjective effects are different from those of psilocybin and psilocin. It is the acetylated form of thepsilocin alkaloid foundin fungi of the genus Psilocybe and also a lower homologue of 4-AcO-MET , 4-AcO-DET , 4-AcO-MiPT and 4-AcO-DIPT .
history
O- acetylpsilocin and several other esters of psilocin were patented on January 16, 1963 by Sandoz AG by Albert Hofmann and Franz Troxler . Despite this fact, psilacetin remains a psychedelic with a limited history of use. It is believed to be a prodrug for psilocin - like psilocybin, which occurs naturally in various mushrooms of the Psilocybe genus . Psilacetin is O -acetylated psilocin, while psilocybin is O -phosphorylated.
presentation
O -acetylpsilocin can be obtained by acetylation of psilocin under alkaline or strongly acidic conditions. O -acetylpsilocin is more resistant than psilocin to oxidation under basic conditions due to its acetoxy group .
pharmacology
In the body is O -Acetylpsilocin of deacetylases / acetyltransferases by a first pass effect and in subsequent passes through the liver to psilocin deacetylated (obviously is Psilacetin also effective by parenteral routes). Its ability to compete for the deacetylase enzymes causes a noticeable loss of cellular heat generating capacity at doses between 0.4 and 0.8 mmol.
Legal position
O -acetylpsilocin is illegal in the UK under the Misuse of Drugs Act 1971 . It is illegal in Italy because it is an ester of a prohibited substance. In Germany, as an ester of DMT , it is also subject to the BtMG .
literature
- Nichols, David; Fescas, Stewart: Improvements to the Synthesis of Psilocybin and a Facile Method for Preparing the O-Acetyl Prodrug of Psilocin , Synthesis , 1999, 6 , pp. 935-938; doi: 10.1055 / s-1999-3490 .
Individual evidence
- ↑ Entry on O-Acetyl Psilocin Fumarate at Toronto Research Chemicals , accessed on July 30, 2017 ( PDF ).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Patent US3075992 : Esters of indoles. Registered on March 28, 1961 , published on January 29, 1963 , applicant: Sandoz, inventor: Albert Hofmann, Franz Troxler.
- ↑ Appendix I BtMG - individual standard. Retrieved January 16, 2020 .