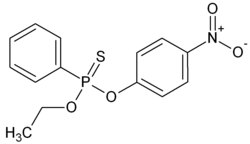
O- ethyl O - (4-nitrophenyl) phenyl thiophosphonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | O- ethyl O - (4-nitrophenyl) phenyl thiophosphonate | |||||||||||||||
| other names |
EPN |
|||||||||||||||
| Molecular formula | C 14 H 14 NO 4 PS | |||||||||||||||
| Brief description |
yellow solid with an aromatic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 323.31 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.27 g cm −3 |
|||||||||||||||
| Melting point |
36 ° C |
|||||||||||||||
| boiling point |
215 ° C (6.5 mbar) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 0.5 mg m −3 (measured as inhalable dust ) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
O -Ethyl- O - (4-nitrophenyl) phenylthiophosphonate ( EPN for short) is an active ingredient for crop protection and an organic chemical compound from the group of thiophosphoric acid esters .
Extraction and presentation
O -Ethyl- O- (4-nitrophenyl) phenylthiophosphonate can be obtained from chlorobenzene in a multistage reaction . In the presence of aluminum chloride, it is reacted with phosphorus trichloride to form phenylthiophosphonyl dichloride and this with sodium ethoxide and the sodium salt of p -nitrophenol to form EPN.
properties
EPN is a flammable yellow solid with an aromatic odor, which is practically insoluble in water.
use
Launched in 1948, EPN is a highly toxic insecticide that is also toxic to warm-blooded animals.
Admission
The active ingredient was never approved in the European Union. In Switzerland, Austria and Germany, no pesticides are permitted that contain EPN as an active ingredient.
Web links
- Poisons Information Monograph (PIM) for Organophosphorus pesticides
Individual evidence
- ↑ a b c d e f g h i Entry on O-Ethyl-O- (4-nitrophenyl) phenylthiophosphonate in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b Ullmann's Agrochemicals, Volume 1 . Viley, 2007, ISBN 978-3-527-31604-5 ( page 546 in the Google book search).
- ↑ Entry on O-ethyl O-4-nitrophenyl phenylphosphonothioate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 2104-64-5 or O-Ethyl-O- (4-nitrophenyl) phenylthiophosphonate ), accessed on November 2, 2015.
- ↑ a b Thomas A. Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5 ( page 284 in the Google book search).
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 12, 2016.

