Nitro anisole
| Nitro anisole | |||||||
| Surname | 2-nitroanisole | 3-nitroanisole | 4-nitroanisole | ||||
| other names |
o -nitroanisole 1-methoxy-2-nitrobenzene |
m -nitroanisole 1-methoxy-3-nitrobenzene |
p -nitroanisole 1-methoxy-4-nitrobenzene |
||||
| Structural formula |
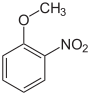
|

|

|
||||
| CAS number | 91-23-6 | 555-03-3 | 100-17-4 | ||||
| PubChem | 7048 | 11140 | 7485 | ||||
| ECHA InfoCard | 100,001,866 | 100.008.255 | 100.002.569 | ||||
| Molecular formula | C 7 H 7 NO 3 | ||||||
| Molar mass | 153.14 g mol −1 | ||||||
| Physical state | liquid | firmly | firmly | ||||
| Brief description | colorless to yellowish or reddish liquid with a characteristic odor |
yellowish crystals |
yellowish gray solid |
||||
| Melting point | 9 ° C | 34-37 ° C | 58 ° C | ||||
| boiling point | 273 ° C |
256.3 ° C |
258–260 ° C above 340 ° C (dec.) |
||||
| density | 1.25 g cm −3 (20 ° C) | 1.23 g cm −3 (25 ° C) | |||||
| Vapor pressure | 0.58 Pa (30 ° C) | ||||||
| solubility | 1.69 g l −1 (30 ° C) | 0.468 g l −1 (20 ° C) | |||||
|
GHS labeling |
|
|
|
||||
| H and P phrases | 350-302 | 302 | 341 | ||||
| no EUH phrases | no EUH phrases | no EUH phrases | |||||
| 201-308 + 313 | 301 + 312 + 330 | 281 | |||||
The nitroanisoles (also methoxynitrobenzenes ) form a group of substances in chemistry that is derived from both anisole and nitrobenzene . The structure consists of a benzene ring with attached methoxy (-OCH 3 ) and nitro group (-NO 2 ) as substituents . Their different arrangement results in three constitutional isomers with the empirical formula C 7 H 7 NO 3 .
presentation
Nitroanisoles can be prepared from the nitrophenols by etherification with dimethyl sulfate .
properties
Due to its symmetry, 4-nitroanisole has the highest melting point.
use
Nitroanisoles are intermediate products (e.g. via anisidines ) in the manufacture of synthetic organic dyes and pharmaceuticals.
safety instructions
2-nitroanisole is suspected to be carcinogenic.
Derivatives
In addition to the singly nitrated anisoles, there are also derivatives with several nitro or other groups that are important.
- 2,4-Dinitroanisole C 7 H 6 N 2 O 5 , is used for the manufacture of explosives
- Trinitroanisole C 7 H 5 N 3 O 7 , is used as an explosive
- Tetranitroanisoles C 7 H 4 N 4 O 9 are also used as explosives
- Methylnitroanisoles C 8 H 9 NO 3
- Other derivatives:
- 2,4-Dinitro-6-methylanisole , CAS number: 29027-13-2
- 2,6-dichloro-4-nitroanisole , CAS number: 17742-69-7
- 2,4-dichloro-6-nitroanisole , CAS number: 37138-82-2
- 2,6-Dimethyl-4-nitroanisole , CAS number: 14804-39-8
Individual evidence
- ↑ a b c d e Entry on 2-nitroanisole in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ a b Data sheet 3-Nitroanisol (PDF) from Merck , accessed on December 27, 2019.
- ↑ a b c d e Entry on 4-nitroanisole in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ H. Félix-Rivera, ML Ramírez-Cedeño, RA Sánchez-Cuprill, SP Hernández-Rivera: Triacetone triperoxide thermogravimetric study of vapor pressure and enthalpy of sublimation in 303-338 K temperature range ; in: Thermochim. Acta , 2011 , 514 , pp. 37-43 ( doi : 10.1016 / j.tca.2010.11.034 ).
- ↑ Data sheet 3-Nitroanisole from Sigma-Aldrich , accessed on December 27, 2019 ( PDF ).
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 209.
- ^ National Institute of Environmental Health Sciences , 13th Report on Carcinogens (RoC): o-Nitroanisole , accessed November 18, 2014.

