Sodium phenylbutyrate
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
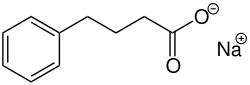
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Sodium phenylbutyrate | |||||||||||||||||||||
| other names |
Sodium (4-phenylbutanoate) |
|||||||||||||||||||||
| Molecular formula | C 10 H 11 NaO 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 186.2 g mol −1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Sodium phenylbutyrate , the sodium salt of 4-phenylbutyric acid , is a chemical compound from the group of sodium salts of aromatic short-chain fatty acids . It is used as drug for the treatment of hyperammonemia (excessive ammonia content in the blood), especially of congenital disorders of the urea cycle as urea cycle disorder , are used.
metabolism
Phenylbutyrate, which is easily absorbed in the intestines, is broken down into phenyl acetate in humans by means of β-oxidation (short-chain acyl-CoA dehydrogenase, SCAD) . Phenylacetate can then be coupled to the amino acid glutamine in the liver , producing phenylacetylglutamine, which is readily water-soluble and can be excreted in the urine. Approximately 80-100% of the conjugation product is excreted in the urine within 24 hours.
A maximum of 2 moles of nitrogen is excreted per 1 mole of phenylbutyrate administered.
properties
In addition to the effect of phenyl butyrate through conjugate formation of its metabolite phenyl acetate in the context of nitrogen elimination from the body, functions both as an enzyme inhibitor (e.g. inhibition of histone deacylase) and as a chaperone are known.
Use in human medicine
The elimination of ammonia by means of phenylbutyrate is used in patients with hyperammonaemia, predominantly in patients with congenital urea cycle defects. Sodium phenylbutyrate is approved as a drug for the treatment of patients with urea cycle disorders under the name Ammonaps . The recommended dosages are:
- 450–600 mg / kg / day for newborns, infants and children <20 kg body weight.
- 9.9–13.0 g / m² / day for children> 20 kg body weight, adolescents and adults.
Pregnancy, breastfeeding, liver and / or kidney insufficiency and hypersensitivity to the active ingredient are contraindications for phenylbutyrate.
The biological half-life of sodium phenylbutyrate is approximately 0.8 hours.
When using sodium phenylbutyrate, it should be noted that it is a sodium compound and therefore quantities of sodium to be taken into account are supplied with the drug (caution with cardiac and renal insufficiency).
Other indications
Sodium phenylbutyrate inhibits in some cases, the tumor growth and shows its effect because of its good liquor marketability especially in brain tumors (eg. As astrocytomas ). However, there was also success in the treatment of prostate and other types of cancer . Attempts to treat spinal muscular atrophy , amyotrophic lateral sclerosis , sickle cell anemia and thalassemia , cystic fibrosis and Huntington's disease , congenital pyruvate dehydrogenase defects and Alzheimer's disease have been carried out or have not yet been completed. For the treatment of rare diseases such as spinal muscular atrophy , citrullinaemia type I (= argininosuccinate synthase deficiency), carbamyl phosphate synthetase deficiency and ornithine transcarbamylase deficiency, sodium phenylbutyrate has the status of an orphan drug .
Side effects
In general, sodium phenylbutyrate is well tolerated. However, side effects can occur after administration of phenylbutyrate. First and foremost is the occurrence of amenorrhoea or dysmenorrhoea, followed by impairment of blood formation, gastrointestinal complaints, fatigue, and the like. a.
If the dosage of phenylbutyrate is too high (e.g. 1000 mg / kg body weight and day), diarrhea, electrolyte changes ( hypokalaemia ) with or without edema formation and metabolic acidosis can occur. A large part of the absorbed phenylbutyrate is then excreted again unmetabolised.
In long-term therapy, it should be noted that the permanent binding of glutamine in the body leads to an increased synthesis of glutamate and glutamine, which is particularly at the expense of the essential branched-chain amino acids, in which a deficiency can develop.
Interactions
If sodium phenylbutyrate and probenecid (a drug used to treat hyperuricemia ) are given at the same time, the excretion of phenylacetylglutamate can be changed. The joint administration of sodium benzoate (also for the treatment of hyperammonaemia) and phenyl butyrate in high doses can lead to the formation of phenylacetyl benzoate and both substances are then no longer available for ammonia elimination.
Finished medicinal products
Ammonaps (EU), Pheburane (EU), Buphenyl (USA)
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b M. L. Batshaw, RB MacArthur, M. Tuchman: Alternative pathway therapy for urea cycle disorders: twenty years later. In: J Pediatr . 138 (1 Suppl), 2001, pp. 46-54.
- ↑ a b c d e f g h Summary of Product Characteristics of Ammonaps (SOBI, Swedish Orphan Biovitrum International) at EMA .
- ↑ SW Brusilow: Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion. In: Pediatr Res . 29, 1991, pp. 147-150.
- ^ AG Kazantsev, LM Thompson: Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. In: Nat Rev Drug Discov . 7, 2008, pp. 854-868. PMID 18827828 .
- ↑ U. Ozcan, E. Yilmaz, L. Ozcan, M. Furuhashi, E. Vaillancourt, RO Smith, CZ Görgün, GS Hotamisligil: Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. In: Science . 313, 2006, pp. 1137-1140.
- ↑ a b T. Iannitti, B. Palmieri: Clinical and experimental applications of sodium phenylbutyrate. In: Drugs R D. 11, 2011, pp. 227-249. PMID 21902286 .
- ↑ R. Ferriero, G. Manco, E. Lamantea, E. Nusco, MI Ferrante, P. Sordino, PW Stacpoole, B. Lee, M. Zeviani, N. Brunetti-Pierri: Phenylbutyrate therapy for pyruvate dehydrogenase complex deficiency and lactic acidosis. In: Sci Transl Med. 5, 2013, p. 175. PMID 23467562 .
- ↑ M. Cuadrado-Tejedor, A. García-Osta, A. Ricobaraza, J. Oyarzabal, R. Franco: Defining the mechanism of action of 4-phenylbutyrate to develop a small-molecule-based therapy for Alzheimer's disease. In: Curr Med Chem . 18, 2011, pp. 5545-5553.
- ↑ AL Ricobaraza, R. Torrijo, R. Franco, A. Garcia-Osta: Phenylbutyrate is a multifaceted drug that exerts neuroprotective effects and reverses the Alzheimer's disease-like phenotype of a commonly used mouse model. In: Curr Pharm Des . 19, 2013, pp. 5076-5084. PMID 23448463 .
- ^ Register of designated Orphan Medicinal Products of the European Commission
- ↑ a b E. Mönch: Defects of the urea cycle - clinical significance and therapy. 2nd Edition. UNI-Med-Verlag, Bremen 2011, ISBN 978-3-8374-1309-0 .