Phosphorus (V) sulfide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phosphorus (V) sulfide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | P 4 S 10 | |||||||||||||||
| Brief description |
yellowish powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 444.54 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.09 g cm −3 |
|||||||||||||||
| Melting point |
286-290 ° C |
|||||||||||||||
| boiling point |
513-515 ° C |
|||||||||||||||
| solubility |
decomposes in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
DFG / Switzerland: 1 mg m −3 (measured as inhalable dust ) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Phosphorus (V) sulfide is a hygroscopic chemical that is mainly used for the synthesis of other products. It usually occurs as a P 4 S 10 molecule and is often incorrectly called di- phosphorus pentasulfide.
presentation
Phosphorus (V) sulfide is obtained by melting the elements white phosphorus and sulfur together at approx. 300 ° C.
properties
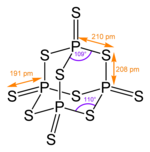 The structure corresponds to that of phosphorus pentoxide .
The structure corresponds to that of phosphorus pentoxide .
The highly flammable and harmful phosphorus (V) sulfide hydrolyzes with water (including air humidity) to form foul-smelling and very poisonous hydrogen sulfide (H 2 S) and phosphoric acid . Phosphorus (V) sulfide is readily soluble in carbon disulfide . P 4 S 10 can catch fire from friction, and contact with moist air can lead to spontaneous combustion.
use
Phosphorus (V) sulfide is used for the synthesis of organic substances containing sulfur and phosphorus, in particular insecticides and zinc dialkyldithiophosphates (additives for lubricants). Phosphorus (V) sulfide can also be used to produce the nerve agent VX (" dual use "). For the preparative production of thiophenes, 1,4-dicarbonyl compounds are also reacted with phosphorus (V) sulfide. Amides can be converted into thionamides by reaction with phosphorus (V) sulfide :
Individual evidence
- ↑ a b c d e f g h i Entry on diphosphorus pentasulfide in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Entry on Diphosphorus pentasulphide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 788.
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 1314-80-3 or phosphorus (V) sulfide ), accessed on November 2, 2015.
- ↑ G. Brauer (Ed.), Handbook of Preparative Inorganic Chemistry 2nd ed., Vol. 1, Academic Press 1963, pp. 565-568.



