phosphorescence
Phosphorescence is the property of a substance to glow in the dark after exposure to (visible or UV) light . The cause of the phosphorescence is the radiating deactivation of the excited atoms and molecules. Alchemists observed this phenomenon as early as the 17th century.
Differentiation from fluorescence
Phosphorescence is a special form of luminescence (cold glow). It differs from the similar phenomenon of fluorescence in that the fluorescence decays quickly after the end of the irradiation, usually within a millionth of a second, whereas in the case of phosphorescence there is an afterglow that can last from fractions of a second to hours. Phosphorescent substances are also known as luminophores because they seem to store light.
- Fluorescence: as soon as the light is off, the fabric no longer glows (e.g. when exposed to UV light - black light in the disco);
- Phosphorescence: glows for many hours in the dark (e.g. emergency exit signs ), see afterglow colors .
phosphorus
A "phosphorescence" was first discovered as long-lasting afterglow in barium sulfide (Bolognese luminous stone) in Bologna in 1602 by Vincentio Casciorolo . Later (1669) Hennig Brand found a similar effect in the white (highly reactive) modification of the chemical element phosphorus (light carrier) he had discovered . Since this afterglow is based on the chemical reaction of atmospheric oxygen with phosphorus, it is, however, a chemiluminescence . The actual phosphorescence describes a quantum physical effect during light excitation.
Colloquially, in the technical field, all materials that can be excited to glow by radiation are referred to as "phosphors". Strictly speaking, these are " luminous pigments " (or luminous dyes ). For example, the inner coating of a Braun tube consists of doped zinc sulfides, which can be excited to glow by electron beams. This inner coating was called “phosphor” on black and white televisions .
Explanation
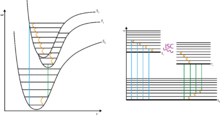
The phenomenon of phosphorescence can with the aid of quantum physics are described and belongs to the group of photophysical processes : If a phosphorescent material with light ( photon absorption of photons) irradiating the appropriate wavelength, as a result that electrons of the phosphor in a higher energy level switch ( Quantum leap ). This excitation from the ground state to an excited state takes place according to the rules of quantum mechanics ( selection rules ), according to which "allowed transitions" have a high probability and thus take place quickly.
The excited state now has several possibilities to release its excitation energy again. If the emission occurs through the emission of a light quantum of an “allowed transition”, one speaks of fluorescence . This process is quantum mechanically allowed, i. H. fast and not associated with the afterglow characteristic of phosphorescence. In addition, energy in the form of vibration energy (heat) can also be released to the environment, with no light emission occurring (internal conversion with subsequent vibration relaxation, see photophysical processes ). As a third possibility, a quantum-mechanically “forbidden” change (see selection rule ) into an excited state can take place, which is referred to as intersystem crossing . As a result, the return to the basic state is “forbidden” according to the selection rules and therefore takes place slowly. If the return takes place under light emission, one speaks of phosphorescence. The excited state acts as a kind of reservoir that is only slowly depopulated. This explains the property of phosphorescence, compared to fluorescence, of being observable over (very) long periods of time (possibly minutes to hours) ("afterglow"). As with fluorescence, deactivation by phosphorescence competes with thermal deactivation, in which energy is given off to the environment in the form of heat (renewed intersystem crossing into a vibration-excited level of the ground state followed by vibration relaxation, see photophysical processes).
In the case of organic compounds, the ground state is usually a singlet state (all electrons are paired). Phosphorescence then corresponds to the transition from the excited triplet state to the singlet ground state. Since the phosphorescence can only inadequately compete with the thermal deactivation of organic compounds in solution, the phosphorescence is mostly only observed at very low temperatures and in solids (crystallized compounds or embedded in solid matrices).
In the case of inorganic compounds ( transition metals , lanthanoids , actinides ) there are often unpaired electrons, so that here the situation with regard to the (spin) multiplicities is more diverse, but it follows the same selection rules.
The transitions and transformations that take place in photophysical processes can be clearly displayed in the Jablonski diagram .
Phosphorescent materials
Phosphorescent materials are usually crystals with a small admixture of a foreign substance that disrupts the lattice structure of the crystal. Usually sulfides of metals of the second group as well as zinc are used and small amounts of heavy metal salts are mixed in (e.g. zinc sulfide with traces of heavy metal salts). In there is an example of a Cu-doped zinc sulfide pigment, the wavelength ranges of the excitation and the radiation as well as the afterglow time course. By fusing boric acid with fluorescein, the doped phosphorescent crystal structures can be made to afterglow with the aid of a UV light source. Europium-doped strontium aluminate, which was developed in 1998 and is offered under the Luminova brand, achieves a long lighting time .
Applications
Postal services
For the automated processing of mail (sorting, applying stamps), different forms of luminescence were used from the second half of the 1950s . For this purpose, graphite strip and phosphor strip prints on postage stamps and fluorescent strips in addition to postal stationery - stamp imprints and phosphorescent and fluorescent paper were used. There were first examples in Great Britain from November 1957 with two graphite stripes printed on the reverse of the stamp. In the Federal Republic of Germany, post offices in the Darmstadt area sold the first postage stamps of the Heuss I and II permanent series with fluorescent paper on August 1, 1960 . In the production of postage stamps , phosphorescent substances have been added to the paper pulp for several decades or the material is subsequently layered. Postage stamps irradiated with UV light then glow in the dark. Postmarking machines can thus recognize where the stamps to be canceled are stuck on the letter and the postmarks are knocked off at the right place. With this method, unfranked letters and postcards can be sorted out and badly forged tokens can be identified.
Security technology
In addition to phosphorescent signs, phosphorescent paints and adhesive tapes are used to mark escape routes. In the case of stairs, the first and last step is marked over the entire width here. Especially in tunnels and hallways that are only used as escape routes, this is an economical and significantly more fail-safe alternative to battery-powered emergency lighting . As early as the Second World War , the walls of many air raid bunkers were painted with phosphorescent colors in order to prevent panic in the otherwise totally dark, often overcrowded bunker rooms in the event of a power failure . A 10-15 cm wide horizontal line at shoulder height was typical in the basement corridor to the shelter. Nowadays, such phosphorescent markings are also often found in underground stations.
Signal character and afterglow
Phosphorescence can also be used well as a signal character . In many cases it is necessary that information is also provided in the dark. Phosphorescent materials are used for luminous hands and dials on clocks and aircraft instruments, on light switches or on some stickers (safety signs, decorative items (stars for the children's room ceiling), car parts, PCs, fishing accessories). Until the 1950s, phosphorescent paints containing radium were common for the pointers and digits of clocks and measuring instruments .
Road markings
In the Netherlands , road markings with phosphorescent colors were used in a pilot project. This should reduce the need for street lights, which leads to additional savings. There is currently a first 500-meter-long section that was completed as a pilot project near the city of Oss (North Brabant Province).
Others
Phosphorescent colors are a stylistic feature in psychedelic art .
Special radar picture tubes (for example the B23G3) were previously used for displays in radar devices . They have a very long afterglow period to show targets until the next round of the radar antenna.
The creation of a silhouette of oneself on a phosphorescent wall with an electronic flash is an attraction in some science centers .
Web links
- University of Jena: phosphorescence
- Peter Bützer: Phosphorescence ( Memento from January 24, 2017 in the Internet Archive )
Individual evidence
- ↑ a b Luminova Glow - Phosphorescent Products. (PDF) Tavco Chemicals Inc., accessed February 8, 2018 (English, product information).
- ↑ a b c Peter Fischer: Phosphorus strips and similar phenomena. In: DBZ - Deutsche Briefmarken-Zeitung , No. 3/2011 of January 28, 2011, page 28
- ^ Ludwig Tröndle: Briefmarkenkunde , Orbis Verlag, ISBN 3-572-00595-7 , page 107
- ↑ Luminous road marking replaces lanterns (heise online)
- ↑ Smart Highway - The intelligent and interactive roads of tomorrow ( Memento of the original from July 20, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Holland Safety Coating. Retrieved May 25, 2018 (nl-NL).
- ↑ radioreinhard.de: B23G3 ( Memento of the original from January 9, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
