Piperophos
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
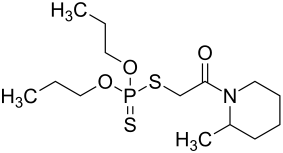
|
||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Piperophos | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 14 H 28 NO 3 PS 2 | |||||||||||||||
| Brief description |
clear, pale yellow, slightly viscous liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 353.48 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| boiling point |
decomposes above 190 ° C |
|||||||||||||||
| solubility |
practically insoluble in water (25 mg l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Piperophos is a 1: 1 mixture ( racemate ) of two isomeric chemical compounds from the group of phosphorodithioates and piperidines . It was introduced as a selective herbicide by Ciba-Geigy in 1969 .
Extraction and presentation
Piperophos can be obtained starting from 2-methylpiperidine by reaction with chloroacetic acid chloride and O, O -dipropyldithiophosphate .
use
Piperophos works by inhibiting the synthesis of long chain fatty acids (VLFCA). It is used against grass weeds in the cultivation of seeds and rice , often in combination with Dimethametryn in order to control weeds at the same time.
Admission
In Germany, Austria and Switzerland, Piperophos is not contained in any approved pesticides.
toxicology
Piperophos is an anti-androgenic endocrine disruptor .
Individual evidence
- ↑ a b c d e Entry on Piperophos. In: Römpp Online . Georg Thieme Verlag, accessed on May 2, 2014.
- ↑ a b Entry on Piperophos in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Entry on S-2-methylpiperidinocarbonylmethyl-O, O-dipropyl phosphorodithioate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 364 ( limited preview in Google Book search).
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 16, 2016.
- ↑ Gunda Viswanath, Shamba Chatterjee, Swati Dabral, Siddharth R. Nanguneri, Gunda Divya, Partha Roy: Anti-androgenic endocrine disrupting activities of chlorpyrifos and piperophos . In: The Journal of Steroid Biochemistry and Molecular Biology . 120, No. 1, 2010, pp. 22-29. doi : 10.1016 / j.jsbmb.2010.02.032 .

