Propetamphos
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
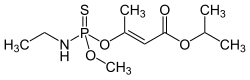
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Propetamphos | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 20 NO 4 PS | |||||||||||||||
| Brief description |
yellowish, oily liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 281.31 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.1294 g cm −3 |
|||||||||||||||
| boiling point | ||||||||||||||||
| solubility |
very heavy in water (0.11 g l −1 at 24 ° C) |
|||||||||||||||
| Refractive index |
1.495 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Propetamphos is a chemical compound from the group of thiophosphoric acid esters . The compound has been used as an insecticide against hygiene pests.
Extraction and presentation
Propetamphos can be prepared from isopropyl acetoacetate and thiophosphoryl trichloride . Their product reacts with methanol and ethylamine to form the end product.
Individual evidence
- ↑ a b Crop Protection Handbook 2014 . 100th edition. MeisterPro, Willoughby, Ohio 2014, pp. 491 .
- ↑ a b c d e f Entry on Propetamphos in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Metabolic Pathways of Agrochemicals, p. 462.
- ↑ Entry on trans-isopropyl-3 - [[(ethylamino) methoxyfosfinothioyl] oxy] crotonate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 381 ( limited preview in Google Book Search).

