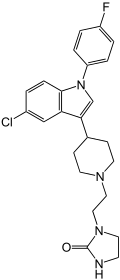
Sertindole
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Sertindole | ||||||||||||||||||
| other names |
1- (2- {4- [5-chloro-1- (4-fluorophenyl) indol-3-yl] piperidin-1-yl} ethyl) imidazolidin-2-one ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 24 H 26 ClFN 4 O | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 440.94 g · mol -1 | ||||||||||||||||||
| Melting point | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Sertindol ( trade name Serdolect ) is a drug that belongs to the group of atypical neuroleptics . It is used to treat schizophrenia .
Pharmacological properties
The drug is distinguished in vitro by a high affinity for D 2 , 5-HT 2 and α 1 receptors . Sertindole also acts as a FIASMA (functional inhibitor of acid sphingomyelinase ).
Sertindole is effective both in the positive symptoms and in the negative symptoms of schizophrenia. Extrapyramidal motor side effects should not be significant. However, there may be a prolongation of the QTc time in the ECG .
Admission status
Sertindole was withdrawn by the manufacturer Lundbeck in 1998 only 16 months after its market launch because of cardiotoxicity , but was re-approved as the second choice medication in 2006 with conditions (in particular EKG controls).
See also
literature
- Andreas Ruß: Medicines pocket 2008. 13th edition. Börm Bruckmeier Verlag, Grünwald 2007, ISBN 978-3-89862-286-8 , p. 251.
- Otto Benkert, Hanns Hippius : Compendium of Psychiatric Pharmacotherapy . 7th completely revised and expanded edition. Springer, Berlin et al. 2008, ISBN 978-3-540-78470-8 , (online edition: ibid. 2009, ISBN 978-3-540-78471-5 , doi : 10.1007 / 978-3-540-78471-5 ).
- Neuroleptic Sertindole (Serdolect) ... readmission not understandable. In: Arznei-Telegramm 8/2006 (37th year), pp. 72–73.
Web links
- Entries in the NIH study registry
- Sertindole Serdolect ® (Promonta). In: Pharmaceutical newspaper online
Individual evidence
- ^ A b The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; P. 1461, ISBN 978-0-911910-00-1 .
- ↑ a b Sertindole data sheet from Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ↑ Entry on sertindole. In: Römpp Online . Georg Thieme Verlag, accessed on May 30, 2014.
- ↑ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer T, Spitzer G, Liedl K, Gulbins E, Tripal P: Identification of novel functional inhibitors of acid sphingomyelinase . In: PLoS ONE . 6, No. 8, 2011, p. E23852. doi : 10.1371 / journal.pone.0023852 .
- ↑ Torsten Kratz, Albert Diefenbacher: Psychopharmacotherapy in old age. Avoidance of drug interactions and polypharmacy. In: Deutsches Ärzteblatt. Volume 116, Issue 29 f. (July 22) 2019, pp. 508-517, p. 512.
- ↑ Kasper S, Möller HJ, Hale A: The European post-marketing observational sertindole study: an investigation of the safety of antipsychotic drug treatment . In: European Archives of Psychiatry and Clinical Neuroscience . 260, No. 1, February 2010, pp. 59-68. PMID 19455275 .
- ↑ Lançon C, Toumi M, Sapin C, Hansen K: The Sertindole Safety Survey: a retrospective analysis under a named patient use program in Europe . In: BMC Psychiatry . 8, 2008, p. 57. PMID 18638382 . PMC 2496904 (free full text).