Silodosin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
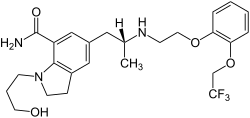
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Silodosin | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 25 H 32 F 3 N 3 O 4 | |||||||||||||||||||||
| Brief description |
white to slightly yellowish white powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 495.53 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
105-109 ° C |
|||||||||||||||||||||
| solubility |
very easily soluble in acetic acid , easily soluble in ethanol and practically insoluble in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Silodosin ( trade names : Urorec and Silodyx , manufacturer: Recordati Pharma GmbH) is a medicinal substance that is used for the symptomatic therapy of benign prostatic hyperplasia (BPH, enlarged prostate gland ).
Pharmacological properties
Mechanism of action (pharmacodynamics)
The active ingredient silodosin is an α1-adrenoceptor antagonist. It works by blocking receptors in the prostate gland, bladder, and urethra called alpha1A adrenoceptors. When these receptors are activated, they cause the muscles that regulate the flow of urine to contract. By blocking these receptors, silodosin allows these muscles to relax, making it easier to pass urine and relieving symptoms of BPH. In direct comparison with tamsulosin , silodosin was found to be significantly more effective in reducing this complex of symptoms, especially in the subgroup of patients who had at least two nocturia episodes per night at the start of the study .
Clinical information
Adverse effects (side effects)
The most common side effects in the clinical trials were retrograde ejaculation (mostly mild, 23.6%), followed by dizziness (2.1%), orthostatic hypotension (1.3%), headache (1.3%), and Diarrhea (1.0%). Occasionally, decreased libido, nausea, dry mouth or erectile dysfunction occurred.
Drug interactions
Silodosin is extensively metabolised, primarily via CYP3A4 , alcohol dehydrogenase, and UGT2B7 . Silodosin is also a substrate for P-glycoprotein . Drugs that inhibit or induce these enzymes and transporters can affect the concentration of silodosin and its active metabolite in plasma.
Alpha blockers : As the safety of the use of silodosin in combination with other α-adrenoreceptor antagonists has not yet been adequately proven, simultaneous use with other α-adrenoreceptor antagonists is not recommended.
CYP3A4 inhibitors : In an interaction study, a 3.7-fold increase in the maximum plasma concentration of silodosin and a 3.1-fold increase in silodosin exposure (i.e. AUC ) were observed with concomitant use of a highly potent CYP3A4 inhibitor ( ketoconazole 400 mg) . Concomitant use with potent CYP3A4 inhibitors (such as ketoconazole, itraconazole or ritonavir ) is not recommended. Was silodosin CYP3A4 inhibitor simultaneously with a moderate such as diltiazem applied, an increase in AUC of silodosin was observed by around 30%, while and plasma half-life were unaffected. This change is of no clinical relevance and no dose adjustment is required.
PDE-5 inhibitors : Minorpharmacodynamic interactions have been observedbetween silodosin and maximum doses of sildenafil or tadalafil . In a placebo-controlled study on 24 volunteers between 45 and 78 years of age who were treated with silodosin, the simultaneous administration of sildenafil 100 mg or tadalafil 20 mg in orthostatic tests (standing versus lying) did not lead to any clinically meaningful mean reductions in systolic or diastolic blood pressure . In subjects over 65 years of age, mean reductions in blood pressure were measured at various times of 5 to 15 mmHg (systolic) and 0 to 10 mmHg (diastolic). Positive orthostatic tests occurred only slightly more frequently during the combined administration; however, nosymptomatic orthostasis or dizziness was observed. Patients taking PDE-5 inhibitors and silodosin concomitantly should be monitored for possible side effects.
Antihypertensive drugs : In the clinical study program, numerous patients received accompanying therapy with antihypertensive drugs (mostly substances that had an effect on the renin-angiotensin system , beta blockers , calcium antagonists and diuretics ). However, the incidence of orthostatic hypotension did not increase. However, concomitant use of antihypertensive drugs should be started with caution and patients should be monitored for possible side effects.
Digoxin : The steady state levels of digoxin, a substrate for P-glycoprotein, were not significantly changed by the simultaneous administration of silodosin 8 mg once daily. No dose adjustment is required.
Admission status
Since January 2010 there have been two main approvals for silodosin in the EU:
- Silodyx
- Urorec
Application limitations
As part of a "Risk Management Plan (RMP)", the marketing authorization holder had to undertake that all ophthalmologists in the Member States in which silodosin is marketed were informed about the connection between silodosin and an intraoperative floppy iris syndrome (IFIS) be informed. The background to this is the observation that IFIS (a variant of small pupil syndrome) was observed during cataract operations in some patients who were treated simultaneously or with a history of α1-blockers. This can lead to an increased rate of complications during the operation.
Studies
- Watson USA: The Evaluation of the Safety of a New Drug for Benign Prostatic Hyperplasia Used for 9 Months . NCT00224133. Clinical Trials
- Watson USA: A New Drug for Benign Prostatic Hyperplasia (BPH) Compared With Placebo . NCT00224120. Clinical Trials
- Watson USA: A New Drug for Benign Prostatic Hyperplasia (BPH) Compared With Placebo . NCT00224107. Clinical Trials
- Recordati: Evaluation of the Efficacy and Safety of Silodosin in the Treatment of the Signs and Symptoms of BPH . NCT00359905. Clinical Trials
- Watson USA: A Study of Silodosin 8 mg Daily for the Treatment of Nocturia in Men With Benign Prostatic Hyperplasia . Clinical Trials
- Recordati: Profile of Silodosin. In: Eur Urol. Suppl 9, 2010, pp. 491-495. PDF (registration required)
Individual evidence
- ^ A b c The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th edition. Merck & Co., Whitehouse Station, NJ, USA 2006, ISBN 0-911910-00-X .
- ↑ a b Datasheet Silodosin, ≥98% (HPLC) from Sigma-Aldrich , accessed on November 5, 2019 ( PDF ).
- ^ S. Morishima, F. Suzuki, H. Yoshiki et al .: Identification of the alpha1L-adrenoceptor in rat cerebral cortex and possible relationship between alpha1L- and alpha1A-adrenoceptors . In: British Journal of Pharmacology . tape 153 , no. 7 , April 2008, p. 1485-1494 , PMID 18223667 , PMC 2437907 (free full text).
- ↑ S. Schilit, KE Benzeroual: Silodosin: a selective alpha1A-adrenergic receptor antagonist for the treatment of benign prostatic hyperplasia . In: Clinical Therapeutics . tape 31 , no. November 11 , 2009, p. 2489-2502 , doi : 10.1016 / j.clinthera.2009.11.024 , PMID 20109995 .
- ↑ Francesco Montorsi: Corrigendum to “Profile of Silodosin” . In: European Urology . tape 59 , no. 2 , February 2011, p. 491–495 , doi : 10.1016 / j.eururo.2010.11.014 .
- ↑ K. Kobayashi, N. Masumori, R. Kato, S. Hisasue, R. Furuya, T. Tsukamoto: Orgasm is preserved regardless of ejaculatory dysfunction with selective alpha1A-blocker administration . In: International Journal of Impotence Research . tape 21 , no. 5 , 2009, p. 306-310 , PMID 19536124 , PMC 2834370 (free full text).
- ↑ Public assessment report (EPAR) of the European Medicines Agency (EMA) for: Silodyx .
- ↑ a b Public Assessment Report (EPAR) of the European Medicines Agency (EMA) on: Urorec .
- ↑ Summary documentation on an amendment to the Drugs Directive (AM-RL): Annex IX - Formation of fixed price groups for alpha-receptor blockers, group 2, in level 2 according to Section 35 (1) SGB V. (PDF; 2.1 MB) G-BA , August 18, 2011, p. 20 , accessed May 27, 2017 .
