Snap tag
A SNAP tag is a protein tag that makes it possible to specifically mark a protein in cells of a cell culture with a fluorescent dye with a high quantum yield .
properties
As standard nowadays, examinations are carried out with protein tags, for example fluorescent proteins, such as e.g. B. GFP (green fluorescent protein) or YFP (yellow fluorescent protein). The methods are relatively easy to perform because the underlying techniques are now very well established (formation of fusion proteins and targeted expression in living cells); on the other hand, the photophysical properties of the proteins are usually not suitable for single-molecule spectroscopy . Compared to commercially available dyes, they have a much lower fluorescence quantum yield and are usually quickly destroyed by excitation with a focused laser beam in the course of photobleaching .
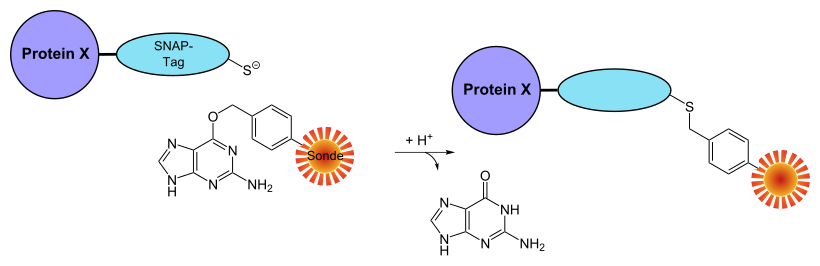
The so-called SNAP protein is a derivative of an enzyme ubiquitous in mammalian cells , O -6-alkylguanine alkyl transferase (AGT), which usually has the task of repairing DNA defects in guanosines in the organism . An alkyl group is transferred from the O 6 -alkylguanine DNA to one of the cysteines of the AGT. The enzyme covalently modified in this way is inactive. The substrate specificity of AGT is low, it also reacts with the nucleobase O 6 -benzylguanine (BG). This property, which also characterizes the SNAP protein, is also used in the course of marking the fusion protein.
Of the partial fragment of the AGT to the mark must, as with all protein tags, first, the DNA sequence in frame in conformity of the protein to be labeled in the DNA sequence inserted are. In addition, any fluorescent dye must be chemically coupled to the SNAP substrate BG-NH 2 . Via an amino group on the BG-NH 2 is NHS - ester of the dye to be used by an S N 2 reaction covalently bound to the substrate. The substrate with the dye then has to diffuse through the membrane into a cell in cell culture. If it meets the SNAP protein there, the ether function contained in the substrate is cleaved by the enzymatic AGT component of the fusion protein , the guanine is released and a direct covalent bond is formed between the protein to be examined and the dye .
Like the CLIP-Tag developed from it, SNAP-Tag is a registered trademark of the biotechnology company New England BioLabs (NEB). The CLIP protein is also a variant of AGT, but specific for benzylcytosine (BC) derivatives. This enables two proteins to be labeled in parallel and independently of one another in one cell.
Reaction scheme
Sources and further reading
- Keppler, A. et al. (2004): Labeling of fusion proteins of O6-alkylguanine-DNA alkyltransferase with small molecules in vivo and in vitro. In: Methods. Vol. 32, pp. 437-444. PMID 15003606
- Keppler, A. et al. (2004): Labeling of fusion proteins with synthetic fluorophores in live cells. In: Proc. Natl. Acad. Sci. USA Vol. 101, pp. 9955-9959. PMID 15226507
- Juillerat, A. et al. (2005): Engineering substrate specificity of O6-alkylguanine-DNA alkyltransferase for specific protein labeling in living cells. In: ChemBioChem Vol. 6, pp. 1263-1269. PMID 15934048
- Brecht, A. & Gibbs, T. (2005): Self Labeling Protein Tags. ( Memento of September 27, 2007 in the Internet Archive ) In: Bioforum. Vol. 2005, No. 6, pp. 50-51.
Web links
- Presentation of SNAP-Tag and CLIP-Tag ( Memento from July 25, 2011 in the Internet Archive ) (NEB)
Individual evidence
- ↑ A. Gautier, A. Juillerat, C. Heinis, IR Corrêa, M. Kindermann, F. Beaufils, K. Johnsson: An engineered protein tag for multiprotein labeling in living cells. In: Chem. Biol. Volume 15, number 2, February 2008, pp. 128-136, doi : 10.1016 / j.chembiol.2008.01.007 , PMID 18291317 .
- ↑ K. Thorn: Genetically encoded fluorescent tags. In: Mol. Biol. Cell. Volume 28, number 7, April 2017, pp. 848-857, doi : 10.1091 / mbc.E16-07-0504 , PMID 28360214 , PMC 5385933 (free full text).