Radiation exchange
As radiation exchange refers to the exchange of energy between two systems or a system and its surroundings by means of electromagnetic waves . The balance of these radiation flows is called the radiation balance .
Exchange of thermal radiation
Heat transfer by radiation
The exchange of thermal radiation is of particular importance . Every body with a temperature above absolute zero emits thermal radiation that is absorbed by other bodies and possibly also by itself . The heat exchange by means of radiation is therefore a process that takes place continuously and everywhere in everyday life. In addition to heat conduction and convection, it represents the third mechanism for heat transport. In a vacuum , radiation exchange is the only possible form of heat transfer (for example for temperature control in spacecraft or for cooling radionuclide batteries in space probes).
Radiative transport differs from the two other heat transport mechanisms in that the observed net heat transport can not only be mathematically divided into two gross heat transports {net heat transport (from 1 to 2) = gross heat transport (from 1 to 2) - gross heat transport (from 2 to 1)}, but this mathematical separation can also be interpreted physically (for example by imagining photons flying from 1 to 2 and vice versa). Or in other words, the thermal radiation not only transports heat in one direction (from warm to cold ), but at the same time the thermal radiation emitted by the colder body also hits the warmer body and can be absorbed by it. This does not contradict the Second Law of Thermodynamics , which in the formulation according to R. Clausius forbids a process in which nothing happens except the transfer of heat from a colder to a warmer body. Because of the mutual visibility of both bodies , heat radiation from the warmer body must also hit the colder one at the same time, so that the radiation transport from cold to warm can never occur in isolation and the requirement of the prohibition ( “nothing happens except ...” ) does not apply. In addition, since more heat radiation is always transferred from the warmer body to the colder body in the net balance than vice versa, the increase in entropy required by the Second Law is ensured in the overall system.
Examples of radiation exchange
- On average, the earth receives a radiation power of 1.74 · 10 17 W from the sun and absorbs about 70% of it, i.e. 1.22 · 10 17 W. Since it is essentially in radiation equilibrium, it has to completely restore the absorbed energy submit. It does this by means of long-wave thermal radiation, which it emits into space on all sides. According to the Stefan-Boltzmann law , it needs an average radiation temperature of about −18 ° C. This corresponds to the mean temperature of the higher atmospheric layers, which serve as radiation surfaces.
- In the event that the earth's atmosphere would not contain any greenhouse gases, the approx. −18 ° C is often given as the surface temperature. But this only applies to the unrealistic case of a uniform temperature of the earth's surface, which would require an ideally heat-conducting globe. For the somewhat more real case that the surface temperature would not be uniform, the average temperature falls according to the Hölder inequality . However, an exact calculation of the same is not possible without precise knowledge of this temperature distribution.
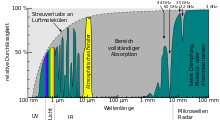
- The ground cannot completely radiate the amount of heat just mentioned into space, because the atmosphere is only partially transparent to the heat radiation . The most important area for the earth's heat balance is highlighted in yellow in the adjacent picture. The other areas marked in blue are either too narrow to allow large amounts of energy to pass through or the approximately 300 K warm earth emits almost nothing in these areas. The atmospheric layers close to the ground absorb most of the thermal radiation emitted by the ground, heat up in the process and in turn give off thermal radiation, partly upwards into higher air layers, partly back down to the ground. This radiation returning to the ground from the air, the so-called atmospheric counter-radiation , has a global mean of around 300 W / m 2 and contributes to the warming of the ground (natural greenhouse effect ).
- In combustion systems, the hot flame gives off most of its heat to its surroundings through flame radiation. Since hot air is a bad heat emitter, the formation of soot particles can be promoted if necessary by choosing suitable combustion conditions or additives, which, as efficient black emitters, support the emission of radiation.
- The amount of heat that a facade surface loses to the environment is determined by the heat transfer coefficient . The heat losses are caused by air convection and heat radiation. When there is no wind, the convective portion of the heat transfer coefficient is on average around 4.5 W / (m 2 · K), the radiation-related portion is around 6.5 W / (m 2 · K). With little air movement, more heat is radiated than is dissipated through the air.
- The feeling of comfort in living spaces depends, among other things, on a balanced exchange of radiation between the person and the environment. During lighter sitting activities, a person generates around 130 W of heat as a result of their metabolic rate . However, according to the Stefan-Boltzmann law, it loses about 900 W of radiant heat (Icl = 0.155 m²K / W, ta = 22 ° C, tr = 22 ° C, vr = 0 m / s). The only reason it does not freeze to death is because a well-tempered environment radiates 770 W of heat, for example. The net heat loss due to radiation is only about 130 W in this example, and that is precisely the loss that is covered by the metabolic rate. The considerable heat radiation exchange is not noticed because gains and losses are almost completely balanced in a comfortable environment.
calculation
The calculation of the amount of energy transferred during the radiation exchange is generally quite complicated, especially when several bodies with complex geometries and complicated radiation properties are involved. The medium between the bodies can also participate in the exchange of radiation through absorption and self-emission. Although, in principle, everything can be calculated using the theory of radiation transport , the exact calculation becomes very extensive and often has little practical value given the various conditions. An easily comprehensible solution is only possible for simple or simplified situations. However, such simple solutions are sufficiently precise for many practical cases.
Simplifications can consist in limiting the number of bodies involved to the most important ones (use of visual factors ) and making simplifying assumptions about the radiation properties of the bodies ( treating them, for example, as Lambert radiators , gray bodies or black bodies ).
Radiation exchange calculations according to the so-called gross method for areas in any enclosed space, which also take into account the tracking of the reflected radiation components, are efficiently possible with a calculation algorithm that can be downloaded free of charge. In order to get even closer to the reality of the radiation exchange, models have also been set up that define a so-called real gray radiator. It takes into account the angular dependence of the emission coefficient.
Simple case: plane-parallel surfaces
Two flat surfaces parallel to one another are considered. They should be very large compared to their distance (i.e. lateral losses should remain negligible) and have different, but in each case uniform and constant absolute temperatures and . Let their absorption / emissivities be and , their reflectance and .
According to the Stefan-Boltzmann law, surface 1 emits radiation of intensity and surface 2 radiation of intensity due to the different temperatures .
First we only consider the radiation from surface 1, which is shown in the graphic:
The entire radiation hits surface 2 with the degree of reflection , which absorbs the portion and reflects the portion on surface 1 with the degree of reflection . This in turn reflects the portion on surface 2, which absorbs the portion . After further reflections, surface 2 absorbs the radiation intensities , etc.
, the sum of all these components, is the total radiation from surface 1 that has been absorbed by surface 2.
Since and are less than 1, the last step followed for the infinite geometric series in brackets from the sum formula .
Insertion of and supplies
The remainder of the radiation originally emitted was absorbed by the surface 1 itself in the course of the multiple reflection.
An analogous observation provides the radiation intensity emitted by surface 2 and absorbed by surface 1 . The net radiation balance is the difference between the radiation intensities absorbed by the two surfaces:
So you get the net radiation power at a panel surface to
with the degree of radiation exchange
- .
The net radiation intensity transmitted from surface 1 to surface 2 is therefore lower than its own thermal emission for two reasons . On the one hand, part of the emitted radiation power is compensated for by the thermal emission emanating from surface 2 . On the other hand, surface 1 receives part of its radiation through surface 2 (regardless of its temperature) reflected back again: is smaller than and . If and / or reduced, i.e. the respective degree of reflection is increased, it quickly becomes very small: the two surfaces hardly exchange any more radiation due to their reflection properties alone. They represent a radiation protection screen, which can prevent thermal radiation very efficiently.
The difference of fourth powers is inconvenient for practical applications. It can be resolved into by applying a binomial formula twice
Define a temperature using
so the net power can simply be written as
| . |
The temperature is between and . If there is not a great difference between the two temperatures, it is often sufficient to use or even or even for the mean of both temperatures .
history
J. Stefan discovered the Stefan-Boltzmann law on the basis of the radiation exchange. In his work from 1879 "On the relationship between thermal radiation and temperature" (session reports of the mathematical and natural science class of the Imperial Academy of Sciences - Vienna 1879) he used the equation for radiation exchange (see above) on page 414, which was his Best described experimental results. Since he did not yet have the current measurement technology, he determined the heat flow via the cooling rate, i.e. H. on the change in temperature over time .
literature
- D. Vortmeyer: VDI-Wärmeatlas - calculation sheets for the heat transfer. 6th edition. Springer-Verlag, 1991, ISBN 3-18-401083-X , Chapter: Calculation of the radiation exchange between several surfaces
- DIN EN ISO 9288: Heat transfer by radiation
Individual evidence
- ^ RM Eisberg, LS Lerner: Physics - Foundations and Applications . McGraw-Hill Int'l, 1982, ISBN 0-07-066268-1 , p. 876.
- ^ A b c d e W. Roedel: Physics of our environment: The atmosphere. 2nd Edition. Springer, Berlin 1994, ISBN 3-540-57885-4 , p. 16.
- ↑ H. Schaube, H. Werner: Heat transfer coefficient under natural climatic conditions . IBP announcement 109 (1986), IRB-Verlag, Stuttgart
- ↑ a b DIN EN ISO 7730: Determination of the PMV and the PPD and description of the conditions for thermal comfort , Berlin, September 1995.
- ↑ B. Glück: Dynamic room model for thermal and thermal physiological evaluation. Report_Raummodell_Teile_A_B_C p. 91 ff.
- ↑ B. luck: heat transfer. P. 160 ff.
- ↑ DIN EN ISO 6946: Components - Thermal resistance and thermal transmittance - Calculation method; Berlin October 2003.



















![M _ {{12}} = \ varepsilon _ {2} \, M_ {1} + \ varepsilon _ {2} \, r_ {1} r_ {2} M_ {1} + \ varepsilon _ {2} \, ( r_ {1} r_ {2}) ^ {2} M_ {1} + \ dots = \ varepsilon _ {2} \, M_ {1} [1 + r_ {1} r_ {2} + (r_ {1} r_ {2}) ^ {2} + \ dots] = M_ {1} {\ frac {\ varepsilon _ {2}} {1-r_ {1} r_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b97177f500d8222a60de14034e4b54295e3c6774)

















