Triethylenetetraminehexaacetic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
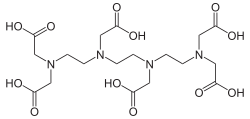
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Triethylenetetraminehexaacetic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 18 H 30 N 4 O 12 | ||||||||||||||||||
| Brief description |
white crystalline solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 494.45 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
232-233 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Triethylenetetraminehexaacetic is a complexing agent from the group of amine carboxylates , in the complexometry for titration of metal ions is used. It is also used to complex radioactive metal ions in radiotherapy with immunoconjugates . Analogs of the TTHA are e.g. B. EDTA and DTPA . A lipophilic derivative is phenyl -TTHA.
TTHA can be coupled to proteins via an alkylamine or a maleimide for molecule labeling . The coupling makes the target molecule more hydrophilic . In connection with some lanthanides , luminescent coupling products can be generated.
literature
- C. De Stefano, A. Gianguzza, D. Piazzese, S. Sammartano: Interactions of diethylenetriaminepentaacetic acid (dtpa) and triethylenetetraaminehexaacetic acid (ttha) with major components of natural waters. In: Analytical and bioanalytical chemistry. Volume 375, Number 7, April 2003, pp. 956-967, doi : 10.1007 / s00216-003-1790-8 , PMID 12707767 .
- MI Prata, MJ Ribeiro, AC Santos, JA Peters, F. Nepveu, CF Geraldes, JJ de Lima: In Vitro and In Vivo Behavior of In Complexes of TTHA, TTHA-Bis (Butylamide) and TTHA-Bis (Glucamide): Stability , Biodistribution and Excretion Studied by Gamma Imaging. In: Metal-based drugs. Volume 5, number 5, 1998, pp. 259-264, doi : 10.1155 / MBD.1998.259 , PMID 18475854 , PMC 2365140 (free full text).
Individual evidence
- ↑ a b c d e data sheet Triethylenetetramine-N, N, N ′, N ′ ′, N ′ ′ ′, N ′ ′ ′ - hexaacetic acid, for complexometry, ≥98.0% from Sigma-Aldrich , accessed on September 1, 2016 ( PDF ).
- ↑ a b B. Achour, J. Costa, R. Delgado, E. Garrigues, CF Geraldes, N. Korber, F. Nepveu, MI Prata: Triethylenetetramine-N, N, N ', N ", N"', N "'-hexaacetic Acid (TTHA) and TTHA-Bis (butanamide) as Chelating Agents Relevant to Radiopharmaceutical Applications. ( Memento of the original from September 2, 2016 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check Original and archive link according to instructions and then remove this note. In: Inorganic chemistry. Volume 37, number 11, June 1998, pp. 2729-2740, PMID 11670409 .
- ↑ SG Gouin, JF Gestin, L. Monrandeau, F. Segat-Dioury, JC Meslin, D. Deniaud: Synthesis and metal complexation properties of Ph-DTPA and Ph-TTHA: novel radionuclide chelating agents for use in nuclear medicine. In: Organic & biomolecular chemistry. Volume 3, Number 3, February 2005, pp. 454-461, doi : 10.1039 / b413758b , PMID 15678183 .
- ↑ KK Bhargava, ZY Zhang, CJ Palestro, SA Acharya: Synthesis of aminobenzyltriethylenetetraaminohexaacetic acid: conjugation of the chelator to protein by an alkylamine linkage. In: Journal of protein chemistry. Volume 18, Number 7, October 1999, pp. 761-770, PMID 10691186 .
- ↑ MS Ali, SM Quadri: Maleimido derivatives of diethylenetriaminepentaacetic acid and triethylenetetraaminehexaacetic acid: their synthesis and potential for specific conjugation with biomolecules. In: Bioconjugate Chemistry . Volume 7, Number 5, 1996 Sep-Oct, pp. 576-583, doi : 10.1021 / bc960051e , PMID 8889020 .
- ↑ LN Krasnoperov, SA Marras, M. Kozlov, L. Wirpsza, A. Mustaev: Luminescent probes for ultrasensitive detection of nucleic acids. In: Bioconjugate Chemistry . Volume 21, Number 2, February 2010, pp. 319-327, doi : 10.1021 / bc900403n , PMID 20085336 , PMC 2839868 (free full text).