Thifluzamide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
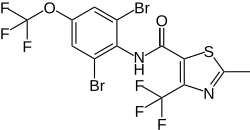
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Thifluzamide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 13 H 6 Br 2 F 6 N 2 O 2 S | ||||||||||||||||||
| Brief description |
white to brown solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 528.06 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.012 g cm -3 |
||||||||||||||||||
| Melting point |
177.9-178.6 ° C |
||||||||||||||||||
| solubility |
poorly soluble in water (1.6 mg l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Thifluzamide is a chemical compound from the group of thiazolecarboxamides .
Extraction and presentation
Thifluzamide can be obtained by condensing 2-methyl-4-trifluoromethyl-5-chlorocarbonylthiazole and 2,6-dibromo-4-trifluoromethoxyaniline.
properties
Thifluzamide is a white to brown solid that is poorly soluble in water.
use
Thifluzamide is used as a systemic fungicide in cereals and rice against various fungal pathogens and as a dressing agent against soil-borne fungi. It is absorbed through the roots and leaves of the treated plants and distributed in them. The active ingredient inhibits succinate dehydrogenase . Thifluzamide was developed by Monsanto in the mid-1990s . It was later sold to Rohm & Haas and is now marketed by Dow AgroSciences .
In Germany, Austria and Switzerland, no pesticides with this active ingredient are permitted.
Individual evidence
- ↑ a b c d e f g h data sheet Thifluzamide, analytical standard at Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ↑ a b Entry on thifluzamide. In: Römpp Online . Georg Thieme Verlag, accessed on February 12, 2015.
- ^ Fluorine and the Environment: Agrochemicals, Archeology, Green Chemistry & Water . Elsevier, 2006, ISBN 978-0-08-046561-6 , pp. 142 ( limited preview in Google Book search).
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 14, 2016.
