Thioxanthene
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
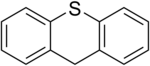
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Thioxanthene | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 13 H 10 S | ||||||||||||||||||
| Brief description |
Crystal needles |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 198.28 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.14 g cm −3 |
||||||||||||||||||
| Melting point |
128.5 ° C |
||||||||||||||||||
| boiling point |
341 ° C |
||||||||||||||||||
| solubility |
soluble in chloroform |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Thioxanthene is a chemical compound from the group of heterocycles and polycyclic aromatic hydrocarbons in which a carbon atom has been replaced by a sulfur atom in the middle of the three rings .
Extraction and presentation
Thioxanthene can be obtained by condensing thiophenol and o- aminobenzoic acid in polyphosphoric acid or by reacting thiophenol with toluene at 450 to 650 ° C.
use
Derivatives of thioxanthene that differ at R 1 and R 2 , like the very similar phenothiazines, are used in medicine as neuroleptics .
Important representatives are Chlorprothixen , Zuclopenthixol / Clopenthixol and Flupentixol . Chlorprothixen has an open side chain , Flupentixol and Zuclopenthixol have a piperazinylalkyl side chain .
Individual evidence
- ↑ a b c d William M. Haynes: CRC Handbook of Chemistry and Physics, 96th Edition . CRC Press, 2015, ISBN 978-1-4822-6097-7 , pp. 512 ( limited preview in Google Book Search).
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons . William Andrew, 2014, ISBN 978-0-323-29060-9 , pp. 322 ( limited preview in Google Book search).
- ↑ a b Data sheet Thioxanthene, European Pharmacopoeia (EP) Reference Standard from Sigma-Aldrich , accessed on November 3, 2016 ( PDF ).
- ↑ Jürgen Jacob: Sulfur Analogues of Polycyclic Aromatic Hydrocarbons (Thiaarenes) Environmental Occurrence, Chemical and Biological Properties . CUP Archive, 1990, ISBN 978-0-521-30120-6 , pp. 1 ( limited preview in Google Book search).
- ↑ B. Müller-Oerlinghausen, Hans-Jürgen Möller, Eckart Rüther: Thioxanthenes in neuroleptic treatment . Springer-Verlag, 2013, ISBN 978-3-642-75194-3 , p. 25 ( limited preview in Google Book search).


