Chlorprothixes
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
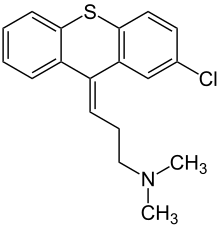
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Chlorprothixes | |||||||||||||||||||||
| other names |
( Z ) -3- (2-chloro-9 H -thioxanthen-9-ylidene) - N , N -dimethylpropan-1-amine ( WHO , IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 18 H 18 ClNS | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 315.86 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
97.5 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Chlorprothixen is a low-potency antipsychotic from the group of thioxanthenes . It is used in the form of tablets , oral solutions or injections for the treatment of restlessness and excitement in special mental and emotional illnesses and of conditions that are characterized by abnormally elevated mood and drive. Since Chlorprothixen can alleviate physical tension, it is sometimes administered as a second-choice drug in addition to analgesics in chronic pain .
history
Chlorprothixen was introduced to the German market in 1959 under the name Truxal .
effect
Chlorprothixen has a very broad spectrum of activity. It has primarily a sedating effect , in higher doses it is anti- psychotic , anti- emetic , local anesthetic , ganglion-blocking , anticholinergic , anti- adrenergic and antihistaminic . The effect comes about through the post- synaptic blockade of dopamine D 1 - and D 2 -, 5-HT 2 -, alpha-1 , H 1 - and muscarinic receptors. Chlorprothixen also acts as a FIASMA (functional inhibitor of acid sphingomyelinase ).
indication
Possible indications are the agitated-anxious or depressive syndrome, mostly in the context of psychotic or bipolar disorders . In contrast to Germany, in Switzerland Chlorprothixen also has a specific and official indication for the treatment of anxiety, restlessness and aggressiveness in alcoholics and toxicomaniac as well as for concomitant medication in chronic pain.
Contraindications
Chlorprothixen must not be used in case of known hypersensitivity to thioxanthenes and in states of deep unconsciousness ( comatose states ). In the presence of a pathologically sad mood ( endogenous depression ), Chlorprothixen may only be used with particular caution.
Side effects
The most common side effects are peripheral vegetative effects such as anticholinergic effects due to the blockade of muscarinic receptors , for example dry mouth, disturbance of accommodation and mydriasis with the risk of a glaucoma attack , constipation , micturition problems with the risk of obstruction of the urine, tachycardia . However, these effects decrease with longer therapy.
Other relevant side effects can be caused by the blockage of peripheral α1-adrenoceptors , i.e. orthostasis with drop in blood pressure or reflex tachycardia, or by direct cardiac effects due to quinidine- like properties such as conduction disorders (PQ / QRS widening).
Central side effects are sedation , drowsiness, delirium (in older patients), increased appetite, weight gain, sleep disorders and epileptic cramps in predisposed persons. Dyskinesias are also possible.
poisoning
Symptoms of poisoning are similar to those of atropine poisoning, i.e. with low doses dry mouth, dry skin, slight bradycardia .
Higher doses lead to thirst, tachycardia , mydriasis , a feeling of dazzling, photophobia, as the poisoning progresses to swallowing disorders (due to the drying up of saliva production), accommodation is no longer possible, intestinal atony , urinary retention , restlessness, confusion, hallucinations , AV block .
In the final stage, the body temperature rises as a result of the inhibition of sweat secretion and the disruption of central regulation; the skin is hot, dry, and red. Finally, the central excitation can turn into somnolence and respiratory paralysis .
Pharmaceutical-chemical information
Chlorprothixen salts are Chlorprothixen acetate , Chlorprothixen citrate and Chlorprothixen hydrochloride . The E isomer ( E ) -3- (2-chloro-9 H -thioxanthen-9-ylidene) - N , N -dimethylpropan-1-amine can occur as an impurity in Chlorprothixenhydrochlorid.
Trade names
Taractan (USA), Truxal (CH, AT), as well as generics (D).
See also
Individual evidence
- ↑ a b c Entry on Chlorprothixen in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b data sheet Chlorprothixene hydrochloride from Sigma-Aldrich , accessed on March 22, 2011 ( PDF ).
- ↑ Entry on Chlorprothixen in Pharmawiki , accessed on January 28, 2017.
- ↑ lumrix.de: Chlorprothixen ( Memento from May 28, 2014 in the Internet Archive ).
- ↑ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer T, Spitzer G, Liedl K, Gulbins E, Tripal P: Identification of novel functional inhibitors of acid sphingomyelinase . In: PLoS ONE . 6, No. 8, 2011, p. E23852. doi : 10.1371 / journal.pone.0023852 .
- ↑ External identifiers or database links for chlorprothixene acetate: CAS number: 58889-16-0, PubChem : 24892861 , ChemSpider : 23346294 , Wikidata : Q27281935 .
- ↑ External identifiers or database links for Chlorprothixencitrat : CAS number: 861959-57-1, PubChem : 24892862 , ChemSpider : 30791766 , Wikidata : Q27269202 .
- ↑ External identifiers or database links for chlorprothixene hydrochloride: CAS number: 6469-93-8, EC number: 229-289-3, ECHA InfoCard: 100.026.627 , PubChem : 5282478 , ChemSpider : 4445622 , Wikidata : Q27254072 .
- ↑ External identifiers or database links to ( E ) -chloroprostheses : CAS number: 4546-35-4, Wikidata : Q89912079 .
- ↑ European Pharmacopoeia 9.0 (2016), p. 2189.