Tralkoxydim
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
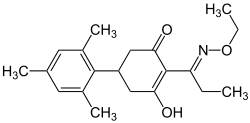
|
|||||||||||||||||||
| Mixture of isomers - simplified structural formula | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tralkoxydim | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 20 H 27 NO 3 | ||||||||||||||||||
| Brief description |
Solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 329.43 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.160 g cm −3 |
||||||||||||||||||
| Melting point |
106 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tralkoxydim is a chemical compound from the groups of cyclohexenones , enols and oximes .
Extraction and presentation
Tralkoxydim can be obtained by a multi-stage reaction of 2,4,6-trimethylbenzaldehyde with acetone , diethyl malonate , pyridine , propionyl chloride and ethoxyamine .
properties
Tralkoxydim is a white to yellowish solid that is practically insoluble in water.
use
Tralkoxydim is a systemic herbicide and was first approved in 1998. It is used against weeds in wheat and barley against oats and other weeds.
Individual evidence
- ↑ a b c d e f g Entry on Tralkoxydim in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d US EPA Pesticides - Fact Sheet: Tralkoxydim dated December 4, 1998, accessed April 20, 2015.
- ↑ Entry on tralkoxydim (ISO); 2- (N-ethoxypropanimidoyl) -3-hydroxy-5-mesitylcyclohex-2-en-1-one in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can use the harmonized classification and expand labeling .
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook: . William Andrew, 1996, ISBN 978-0-8155-1853-2 , pp. 159 ( limited preview in Google Book search).
- ↑ David Herd Hutson: Metabolic Pathways of Agrochemicals: Herbicides and plant growth regulators: . Royal Society of Chemistry, 1998, ISBN 978-0-85404-494-8 , pp. 237 ( limited preview in Google Book search).


