Transcription-Translation Feedback Loop
The transcription-translation feedback loop ( TTFL ) to German transcription - translation -Rückkopplungsschleife , is a cellular model to explain circadian rhythms in behavior and physiology . The TTFL is preserved in many ways and regulates itself automatically. The transcription of certain genes , the so-called clock genes (“clock” genes), is regulated by their own protein products.
discovery
Circadian rhythms have been known for centuries. As early as 1729 , the French astronomer Jean Jacques d'Ortous de Mairan noticed the periodic movement of the mimosa plant leaves within 24 hours. However, science has only recently begun to uncover the cellular mechanisms responsible for driving the circadian rhythms observed. The cellular basis of circadian rhythms is supported by the fact that rhythms have been observed in unicellular organisms.
From the 1970s, experiments by Ron Konopka et al. in which methods of forward genetics (English: forward genetics ) were used to induce mutations, that species of Drosophila melanogaster with changed genes, the so-called period genes (per), also had a changed periodicity. As genetic and molecular biological experimental tools improved, researchers identified additional genes that are responsible for maintaining normal rhythmic behavior. This gave rise to the concept that internal rhythms are modified by a small subset of core clock genes. Paul Hardin et al . In 1990, the first group to suggest that the mechanism that drives these rhythms is a negative feedback loop .
Subsequent important discoveries confirmed this model. In particular, the experiments led by Thomas K. Darlington and Nicholas Gekakis in the late 1990s identified CLOCK proteins and characterized their methods in Drosophila and mice, respectively. The TTFL model emerged from these experiments and has now become the dominant paradigm for explaining circadian behavior for a large number of species.
General mechanisms of the TTFL
The TTFL is a negative feedback loop in which clock genes are regulated by their protein products. In general, TTFL comprises two feedback loops: positive regulatory elements that promote transcription and protein products that suppress transcription. If a positive regulatory element to a clock - promoter binds the transcription progresses, leading to the formation of an mRNA leads -Transkripts. This is followed by translation, resulting in a protein product. There are characteristic delays between mRNA transcript accumulation, protein accumulation and gene silencing due to translational dynamics , post-translational modification , protein dimerization and intracellular transport to the nucleus .
Cross-species proteins that participate in TTFL contain common structural motifs such as PAS domains , which are involved in protein-protein interactions , and bHLH domains , which are involved in DNA binding.
Models
The presence of the TTFL is highly conserved in all animal species. However, many of the actors involved in the process have changed within different species over the course of development. When comparing plants, animals, fungi, and other eukaryotes, there are differences in the genes and proteins involved in TTFL. This suggests that a "clock" that follows the TTFL model has evolved several times during its existence.
| Drosophila melanogaster | |
|---|---|
| Positive regulators | CYC, clock |
| Negative regulators | TIM, PER |
| Mammals | |
| Positive regulators | BMAL1, CLOCK |
| Negative regulators | PER1, PER2, CRY1, CRY2 |
| Neurospora | |
| Positive regulators | WC-1, WC-2 |
| Negative regulators | FRQ |
Drosophila melanogaster
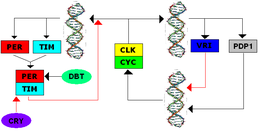
The TTFL was first discovered in Drosophila and the system has several similarities with the TTFL of mammals. The transcription of the clock genes period (per) and timeless (tim) is initiated when the positive protein elements CYCLE (dCYC) and CLOCK (dCLK) form a heterodimer and bind E-Box promoters, which initiates transcription. The protein product TIM is broken down during the day; Exposure facilitates the binding of the protein CRY to TIM, whereby TIM is ubiquitinated and eventually degraded. On the night of TIM and PER heterodimers can form and slowly in the cytoplasm accumulate where PER by the kinase DBT (double-time protein) phosphorylates is. The post-transcriptional modification of several phosphate groups serves to break down the complex and to facilitate the localization of the complex in the cell nucleus. In the cell nucleus, the PER-TIM dimer binds to the CYC-CLK dimer, which releases the CYC-CLK dimer from the E-boxes and inhibits transcription. Once PER and TIM degrade, CYC-CLK dimers can rebind the E-boxes to initiate transcription and close the negative feedback loop.
Secondary feedback loops interact with the primary feedback loop. CWO ( Clockwork Orange Drosophila protein ) binds the E-boxes as a direct competitor of CYC-CLK and thus inhibits transcription. PDP1ε ( par domain protein 1ε ) is a feedback activator and VRI ( vrille ) is a feedback inhibitor of the clock promoter; their expression is activated by dCLK-dCYC. The ecdysone-induced protein 75 (E75) inhibits clock expression and is activated in a time-dependent manner per transcription. All secondary loops reinforce the primary TTFL.
Cryptochrome in Drosophila is a blue light photoreceptor that triggers the breakdown of TIM and indirectly causes the clock phase to be reset and the expression of the per gene to be promoted again.
Mammals
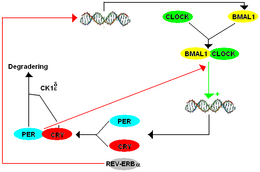
The mammalian TTFL model contains many components that are homologues of those found in Drosophila . The mammalian TTFL works so that BMAL1 forms a heterodimer with CLOCK to initiate transcription of mPer and cryptochrome (cry). There are three paralogues or historically similar genes that appear today as a duplication of the period gene in mammals listed as mPer1 , mPer2, and mPer3 . There are also two paralogues of cryptochrome in mammals. PER and CRY proteins form a heterodimer and the phosphorylation of PER by CK1δ ( casein kinase I isoform delta ) and CK1ε ( casein kinase 1 isoform epsilon ) regulates the localization of the dimer to the cell nucleus. In the nucleus, PER-CRY negatively regulates the transcription of its related genes by binding BMAL1-CLOCK and releasing them from the E-box promoter.
Although the mPer paralogues work together as functional orthologues of dPer , they each have a special function. mPer1 and mPer2 are necessary for the “clock function” in the brain, while mPer3 only plays a recognizable role in the circadian rhythm of peripheral tissues. When the gene knockout of mPer1 or mPer2 causes a change in the free- running period ( τ ), mPer1 with a shorter period and mPer2 with a longer period compared to the original period, before an arrhythmia finally occurs. Similarly, mCry1 knockouts lead to a shortened period and mCry2 knockouts to a lengthened period, with a double mCry1 or mCry2 knockout leading to arrhythmia.
There are also secondary loops in mammals, although these are more complex than in Drosophila . As with CWO in Drosophila, Dec1 ( Deleted in esophageal cancer 1 ) and Dec2 ( Deleted in esophageal cancer 2 ) suppress mPer expression by binding E-boxes, which prevents the CLOCK-BMAL1 protein complex from attaching to the E-boxes to tie. The Rev-erb and ROR ( RAR-related orphan receptor ) receptors play a role similar to PDP1ε and VRI in Drosophila , except that they regulate the CLOCK binding partner BMAL1 instead of directly regulating CLOCK. DBP ( D site of albumin promoter (albumin D-box) binding protein ) and E4BP4 ( E4 promoter-binding protein 4 ) bind to the D-box promoter sequence in order to regulate mPer expression.
The way in which these genes are related to Drosophila melanogaster is seen in the function of each individual gene and in the way in which they have changed evolutionarily. BMAL1 is an ortholog from CYCLE. This means that BMAL1 and CYCLE appear to have a common history, but occur in different species. Another example of the parallels between Drosophila melanogaster and mammals can also be found in cry and mPer , since they are functional orthologues of per and tim .
Neurospora
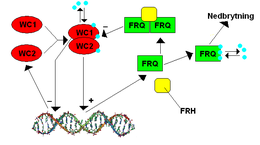
The frequency (frq) gene in Neurospora was identified in 1979 by JF Feldman and his colleagues as the second known clock gene at the time . FRQ was first cloned in 1989 by CR McClung and his colleagues. This gene was of particular interest because its expression is very complex compared to other known microbial genes.
Two positive regulatory proteins, Wc-1 ( white collar 1 protein ) and Wc-2 ( white collar 2 protein ), bind the frq promoter, which is called the clock box , during the late subjective night to activate transcription. Light is also important to induce FRQ expression. WC-1 is a photopigment, and light enables WC-1 and WC-2 to bind to another promoter called a proximal light-response element (PLRE). The FRQ protein negatively regulates the activity of WC-1 and WC-2. Several kinases (CK1-, CK2- and Checkpoint kinase 2-like ) and phosphatases (PP1 and PP2A) regulate the ability of FRQ to move into the cell nucleus and the stability of FRQ, WC-1 and WC-2.
Arabidopsis
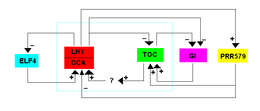
The first TTFL model was proposed for Arabidopsis in 2001 and included three MYB transcription factors , LHY ( Late Elongated Hypocotyl ), CCA1 ( Circadian Clock Associated 1 ) and TOC1 ( Timing of CAB expression 1 ). CCA1 and LHY are expressed in the morning and interact with each other to suppress the expression of TOC1. The CCA1 and LHY expression decreases in the dark, so that TOC1 is expressed and the CCA1 and LHY expression is negatively regulated. CCA1 and LHY can also bind to their own promoter to suppress their own transcription.
A second loop consists of PRR9, PRR7 and PRR5, which are all homologues of TOC1 and suppress CCA1 and LHY expression. These PRR genes are directly suppressed by LHY and TOC1. These genes are also regulated by the Evening Complex (EC), which consists of LUX ARRHYTHMO (LUX), EARLY FLOWERING 3 (ELF3) and EARLY FLOWERING 4 (ELF4). LUX is a transcription factor with a function similar to MYB, while ELF3 and ELF4 are core proteins whose functions are unknown. The evening complex indirectly promotes the expression of LHY and CCA1, whereby the transcription of its own components is suppressed. Since this model consists of two inhibitions that lead to activation, it is also known as a repressilator .
A recently discovered loop includes the reveille family of genes that are expressed in the morning and induce the transcription of "evening genes" such as PRR5, TOC1, LUX and ELF4. Once the resulting proteins are translated, PRR9, PRR7 and PRR5 suppress the RVE8 gene. RVE8 also interacts with the “morning components” LNK1, 2, 3 and 4 (LNK: night light-inducible and clock-regulated ), with LNKs either antagonizing or co-activating the RVE8 gene .
Although the GIGANTEA (GI) protein is not known to be a core component of the Arabidopsis -TFL model, it is suppressed by CCA1, LHY, and TOC1. In addition, GI activates CCA1 and LHY expression.
Cyanobacteria
Studies on the “cyanobacteria clock” led to the discovery of three essential clock genes: KaiA , KaiB and KaiC . Initially, it was thought that these proteins were similar to the TTFL model proposed for eukaryotes , as there is a daily pattern in terms of mRNA and protein abundance and degree of phosphorylation, as well as negative feedback from proteins on their related genes, a reset of the clock phase in response to KaiC overexpression and modified Kai activity through interactions.
Each of these results agreed with the understanding of the TTFL at the time. However, later studies concluded that post-translational modifications such as phosphorylation are more important for clock control. When promoters for the Kai proteins were replaced by unspecific promoters, there was no break in the central feedback loop, as would be expected if there was an inhibition by the feedback of the proteins to their specific promoters. As a result, the TTFL model for cyanobacteria has been found to be largely inaccurate. Transcription regulation is not the central process that drives the rhythm of cyanobacteria. Although regulation of transcription and translation are in place, they have been viewed as effects of the "clock" rather than necessary for clock function.
Alternatives to the TTFL model
Post-translational feedback loops (PTFL) have also been uncovered, which are involved in the regulation of clock genes and often work in conjunction with the TTFL model. In both mammals and plants, post-translational modifications such as phosphorylation and acetylation regulate the frequency and / or the activity of clock genes and proteins. For example, the degrees of phosphorylation of TTFL components have been shown to vary rhythmically. These post-translational modifications can serve as degradation signals, binding regulators and signals for the recruitment of additional transcription factors.
In particular, cyanobacteria show rhythmic changes in phosphorylation after 24 hours in a feedback loop independent of transcription and translation: circadian rhythms of phosphorylation are observed when the Kai proteins of the feedback loop are placed in a test tube with ATP , regardless of other cellular mechanisms. It is widely accepted that this three protein post-translational system is the main oscillator necessary and sufficient to keep the daily rhythm going.
In addition to the Kai system in cyanobacteria, it has been shown that peroxiredoxin oxidation occurs independently of transcription and translation in both red mammalian blood cells and in Ostreococcus tauri cells of the algae. It has been found that this system is preserved in many organisms. It is not clear whether the peroxiredoxin system interacts with TTFL-based clocks or is itself part of a new PTFL-based clock. However, both findings imply that in some organisms or cell types, PTFL is sufficient to control the circadian rhythm.
Individual evidence
- ^ D. Mergenhagen: Circadian rhythms in unicellular organisms. In: Current topics in microbiology and immunology. Volume 90, 1980, pp. 123-147, doi : 10.1007 / 978-3-642-67717-5_6 , PMID 6775877 (review).
- ↑ Lisa Wulund, Akhilesh B. Reddy: A brief history of circadian time: The emergence of redox oscillations as a novel component of biological rhythms. In: Perspectives in Science. 6, 2015, p. 27, doi : 10.1016 / j.pisc.2015.08.002 .
- ^ MH Hastings, ES Maywood, JS O'Neill: Cellular circadian pacemaking and the role of cytosolic rhythms. In: Current Biology . Volume 18, Number 17, September 2008, pp. R805-R815, doi : 10.1016 / j.cub.2008.07.021 , PMID 18786386 (review).
- ↑ JC Dunlap, JJ Loros, Y. Liu, SK Crosthwaite: Eukaryotic circadian systems: cycles in common. In: Genes to cells: devoted to molecular & cellular mechanisms. Volume 4, Number 1, January 1999, pp. 1-10, PMID 10231388 (review).
- ^ AS Loudon: Circadian biology: a 2.5 billion year old clock. In: Current Biology . Volume 22, Number 14, July 2012, pp. R570-R571, doi : 10.1016 / j.cub.2012.06.023 , PMID 22835791 .
- ^ T. Yoshii, C. Hermann-Luibl, C. Helfrich-Förster: Circadian light-input pathways in Drosophila. In: Communicative & integrative biology. Volume 9, number 1, 2016 Jan-Feb, p. E1102805, doi : 10.1080 / 19420889.2015.1102805 , PMID 27066180 , PMC 4802797 (free full text) (review).
- ↑ a b c d e f g T. S. Andreani, TQ Itoh, E. Yildirim, DS Hwangbo, R. Allada: Genetics of Circadian Rhythms. In: Sleep medicine clinics. Volume 10, number 4, December 2015, pp. 413-421, doi : 10.1016 / j.jsmc.2015.08.007 , PMID 26568119 , PMC 4758938 (free full text) (review).
- Jump up ↑ JC Dunlap, JJ Loros, HV Colot, A. Mehra, WJ Belden, M. Shi, CI Hong, LF Larrondo, CL Baker, CH Chen, C. Schwerdtfeger, PD Collopy, JJ Gamsby, R. Lambreghts: A circadian clock in Neurospora: how genes and proteins cooperate to produce a sustained, entrainable, and compensated biological oscillator with a period of about a day. In: Cold Spring Harbor symposia on quantitative biology. Volume 72, 2007, pp. 57-68, doi : 10.1101 / sqb.2007.72.072 , PMID 18522516 , PMC 3683860 (free full text) (review).
- ^ A b c d S. E. Sanchez, SA Kay: The Plant Circadian Clock: From a Simple Timekeeper to a Complex Developmental Manager. In: Cold Spring Harbor perspectives in biology. Volume 8, number 12, December 2016, p., Doi : 10.1101 / cshperspect.a027748 , PMID 27663772 , PMC 5131769 (free full text) (review).
- ^ CH Johnson, T. Mori, Y. Xu: A cyanobacterial circadian clockwork. In: Current Biology . Volume 18, number 17, September 2008, pp. R816-R825, doi : 10.1016 / j.cub.2008.07.012 , PMID 18786387 , PMC 2585598 (free full text) (review).
- ↑ Ben Sheredos: Scientific Diagrams as Traces of Group-Dependent Cognition: A Brief Cognitive-Historical Analysis. In: Cognitive Science Society. eScholarship, 2013, accessed October 28, 2019 .
- ^ S. Kojima, DL Shingle, CB Green: Post-transcriptional control of circadian rhythms. In: Journal of cell science. Volume 124, Pt 3Februar 2011, pp. 311-320, doi : 10.1242 / jcs.065771 , PMID 21242310 , PMC 3021995 (free full text) (review).
- ↑ JM Hurley, JJ Loros, JC Dunlap: Circadian Oscillators: Around the Transcription-Translation Feedback Loop and on to Output. In: Trends in Biochemical Sciences . Volume 41, number 10, 10 2016, pp. 834-846, doi : 10.1016 / j.tibs.2016.07.009 , PMID 27498225 , PMC 5045794 (free full text) (review).
- ^ SA Brown, E. Kowalska, R. Dallmann: (Re) inventing the circadian feedback loop. In: Developmental cell. Volume 22, Number 3, March 2012, pp. 477-487, doi : 10.1016 / j.devcel.2012.02.007 , PMID 22421040 (review).