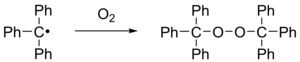Triphenylmethyl radical
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Triphenylmethyl radical | ||||||||||||
| other names |
Trityl radical |
||||||||||||
| Molecular formula | C 19 H 15 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 243.33 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
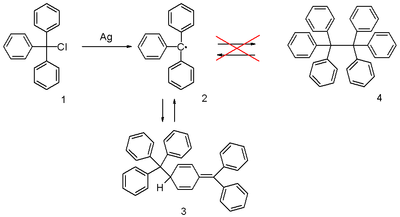
The triphenylmethyl radical (often abbreviated as trityl radical ) is a stabilized radical and the first radical to be described in organic chemistry . It can be obtained by homolysis of triphenylmethyl chloride 1 by a suitable metal such as silver or zinc in benzene or diethyl ether . The radical 2 is in chemical equilibrium with the quinone-like dimer 3 (3-triphenylmethyl-6-diphenylmethylidene-1,4-cyclohexadiene, also known as Gomberg's dimer ). The concentration of the radical in benzene is 2%.
Solutions of the radical are yellow , the color increasing in intensity with increasing temperature . In accordance with Le Chatelier's principle, this is due to an equilibrium shift on the side of the radical.
If the triphenylmethyl radical is exposed to air , it quickly oxidizes to peroxide and the corresponding solutions discolor.
It reacts with iodine to form triphenylmethyl iodide .
The radical was discovered in 1900 by Moses Gomberg at the University of Michigan . He had tried to synthesize hexaphenylethane by means of a Wurtzian synthesis from triphenylmethlchlorid and zinc in benzene. He found that the product obtained had a significantly higher reactivity towards oxygen and iodine than expected. The structure discovered in this way was later used in the development of ESR spectroscopy and was confirmed by this.
The correct, quinone-like structure was proposed for the first time as early as 1904, but was rejected by the general public of chemists against hexaphenylethane 4 . It then took until 1968 until it was rediscovered and confirmed by researchers at the Vrijen Universiteit Amsterdam through NMR spectroscopic experiments.
While the triphenylmethyl radical forms a quinoin-like dimer, a dimer with a hexaphenylethane-like structure could be detected for differently substituted derivatives . X-ray crystallographic examinations showed a length of 1.67 Å for the central C – C bond in hexakis (3,5-di- t -butylphenyl) ethane. This is very long compared to a typical single bond at 1.54 Å. Theoretical calculations at a high level suggest that the reason for the stability of this sterically hindered molecule is due to London dispersion interactions between the tert-butyl substituents. The resulting minimum potential is missing in the unsubstituted molecule. Derivatives with other substitution patterns were found in the form of the quinoinarite dimer.
See also
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ J. March: Advanced Organic Chemistry . John Wiley & Sons, March 11, 1985, ISBN 0-471-88841-9 .
- ↑ M. Gomberg : An instance of trivalent carbon: triphenylmethyl . In: Journal of the American Chemical Society . 22, No. 11, 1900, pp. 757-771. doi : 10.1021 / ja02049a006 .
- ^ M. Gomberg : On trivalent carbon . In: Journal of the American Chemical Society . 23, No. 7, 1901, pp. 496-502. doi : 10.1021 / ja02033a015 . (Note: Radical is also known as a cadicle .)
- ^ M. Gomberg : On trivalent carbon . In: Journal of the American Chemical Society . 24, No. 7, 1902, pp. 597-628. doi : 10.1021 / ja02021a001 .
- ^ SI Weissman, John C. Sowden: Electron distribution in triphenylmethyl: Hyperfine structure of the paramagnetic resonance absorption of (C 6 H 5 ) 3 C 13 * . In: Journal of the American Chemical Society . 75, No. 2, 1953, p. 503. doi : 10.1021 / ja01098a522 .
- ↑ J. Sinclair, D. Kivelson: Electron spin resonance studies of substituted triphenylmethyl radicals . In: Journal of the American Chemical Society . 90, No. 19, 1968, pp. 5074-5080. doi : 10.1021 / ja01021a004 .
- ^ ESR spectrum of the triphenylmethyl radical . School of Chemistry, University of Bristol.
- ↑ JM McBride: The hexaphenylethane riddle . In: Tetrahedron . 30, No. 14, 1974, pp. 2009-2022. doi : 10.1016 / S0040-4020 (01) 97332-6 .
- ↑ H. Lankamp, W. Th. Nauta, C. MacLean: A new interpretation of the monomer-dimer equilibrium of triphenylmethyl- and alkyl-substituted-diphenyl methyl-radicals in solution . In: Tetrahedron Letters . 9, No. 2, 1968, pp. 249-254. doi : 10.1016 / S0040-4039 (00) 75598-5 .
- ↑ Errol G Lewars: Hexaphenylethane . In: Modeling Marvels . Springer Netherlands, Dordrecht 2008, ISBN 978-1-4020-6972-7 , pp. 115–129 , doi : 10.1007 / 978-1-4020-6973-4_8 ( springer.com [accessed September 25, 2019]).
- ↑ Grimme, Peter R. Schreiner: Steric crowding can stabilize a labile molecule: Solving the hexaphenylethane riddle . In: Angewandte Chemie International Edition . 50, No. 52, 2011, pp. 12639-12642. doi : 10.1002 / anie.201103615 .
- ↑ Y. Uchimura, T. Takeda, R. Katoono, K. Fujiwara, T. Suzuki: New Insights into the Hexaphenylethane Riddle: Formation of an α, o -Dimer. . In: Angewandte Chemie International Edition . 54, No. 13, 2015, pp. 4010-4013. doi : 10.1002 / anie.201500122 .