Trityl chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Trityl chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 19 H 15 Cl | |||||||||||||||
| Brief description |
light yellow powder with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 278.78 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
109-112 ° C |
|||||||||||||||
| boiling point |
230–235 ° C (at 27 hPa ) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Trityl chloride is a reactive organic chemical substance that is used as a protective group for primary alcohols .
Manufacturing
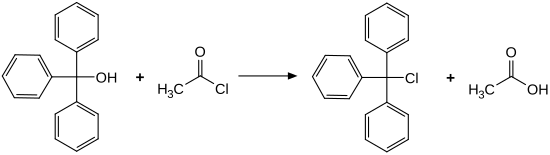
Trityl chloride is commercially available. To produce it, triphenylmethanol is reacted with acetyl chloride . Alternatively, Friedel-Crafts alkylation of benzene with carbon tetrachloride yields a salt of trityl chloride and aluminum chloride , which upon hydrolysis yields trityl chloride.
properties
The most important thermodynamic properties are listed in the following table:
| property | Formula symbol | Value (remark) |
|---|---|---|
| Standard enthalpy of formation | Δ f H 0 (s) | 183 kJ mol −1 |
| Enthalpy of combustion | Δ c H 0 (s) | −9826 kJ mol −1 |
| Heat capacity | c p | 367.27 J mol −1 K −1 (as a solid at 25 ° C) |
| Enthalpy of fusion | Δ f H 0 | 27.9 kJ mol −1 (at the melting point) |
| Entropy of fusion | Δ f S 0 | 74.1 kJ mol −1 (at the melting point) |
use
To synthesize trityl ethers , an alcohol is reacted with trityl chloride (Ph 3 CCl, abbreviated as TrCl) in the presence of a base (e.g. pyridine ). For steric reasons, only primary alcohols are etherified with trityl chloride and, in the case of monosaccharides , for example, it is possible to selectively protect the hydroxyl group at C-6 in addition to all others.
In addition, the triphenylmethyl radical can be prepared from trityl chloride.
Individual evidence
- ↑ a b c d e data sheet Trityl chloride, purum at Sigma-Aldrich , accessed on May 19, 2017 ( PDF ).
- ↑ a b c Entry on chlorotriphenylmethane in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ^ WE Bachmann, CR Hauser, Boyd E. Hudson, Jr .: Triphenylchloromethane In: Organic Syntheses . 23, 1943, p. 100, doi : 10.15227 / orgsyn.023.0100 ; Coll. Vol. 3, 1955, p. 841 ( PDF ).
- ↑ a b Schmidlin, MJ: Recherches chimiques et thermochimiques sur la constitution des rosanilines in Ann. Chim. Phys., 1906, 1, 195-256.
- ↑ a b c Naoki, M .; Seki, M .; Kugo, H .; Saito, F .; Taioka, T .: Dielectric relaxation in supercooled triphenylchloromethane and intrinsic factor determining mobility in molecular liquids in J. Phys. Chem. 95 (1991) 5628-5633.
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren, Peter Wothers: Organic Chemistry . Oxford University Press, 2001, p. 1370. ISBN 978-0-19-850346-0 .
- ↑ P. Collins, R. Ferrier: Monosacharides - Their Chemistry and their Roles in Natural Products. Wiley West Sussex 1995, ISBN 0-471-95343-1 .
- ↑ PJ Kocienski: Protecting Groups. Georg Thieme Verlag, Stuttgart 1994, ISBN 3-13-135601-4 .
- ↑ Jerry March, 1929–1997 .: Advanced organic chemistry: reactions, mechanisms, and structure . 3rd ed. Wiley, New York 1985, ISBN 0-471-88841-9 .