Tyrosinase
| Tyrosinase | ||
|---|---|---|
| Properties of human protein | ||
| Mass / length primary structure | 511 amino acids | |
| Secondary to quaternary structure | Membrane protein | |
| Cofactor | 2 copper | |
| Isoforms | 2 | |
| Identifier | ||
| Gene name | TYR | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.14.18.1 , dioxygenase | |
| Response type | oxidation | |
| Substrate | L-tyrosine + L-dopa + O 2 | |
| Products | L-dopa + dopaquinone + H 2 O | |
| Occurrence | ||
| Homology family | Tyrosinase | |
| Parent taxon | Creature | |
Tyrosinase is a copper- containing enzyme that prevents the oxidation of phenols , e.g. B. tyrosine , catalyzed . It is widespread in almost all living things.
In animals and humans, tyrosinase (together with the enzymes Tyrp1 and Dct ) is involved in the synthesis of melanin on the membrane of melanocytes and is therefore essential for protection against UV radiation . In some of the organisms with albinism , the enzyme is changed or completely absent. The tyrosinase in plants has additional functions and is called polyphenol oxidase .
Melanogenesis
The production of tyrosinase increases with increased UVB radiation. Then the following chemical reactions take place on the cell membrane of melanoblasts .
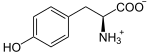
Tyrosine is first oxidized to L-Dopa .
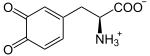
L-Dopa is further oxidized to dopachinone , which is independently oxidized in two steps to dopachrome . From here, a two-step reaction path to melanin is possible with the help of the enzymes TYRP1 and DCT . The tyrosinase can also produce melanin from dopachrome itself:
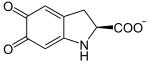
Dopachrome is decarboxylated to 5,6-dihydroxyindole (DHI) and rearranged.
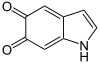
DHI is oxidized to indole-5,6-quinone , which with the help of oxygen ultimately polymerizes to melanin (not shown).
It follows that tyrosinase is essential for melanin synthesis, while a deficiency of Tyrp1 or Dct can be more or less compensated for by tyrosinase. The amount of melanin produced is reduced in each case, so here is a simple explanation for genetic variations in the color of skin, eyes and hair.
ortho- vanillin is a weak and monobenzone a strong inhibitor of tyrosinase.
pathology
Mutations in the TYR gene are responsible for oculocutaneous albinism type 1 (OCA1). A variant of Waardenburg syndrome type 2 results from an interaction of a mutation in the tyrosinase locus (R402Q) with a mutation in the Mitf gene .
Albinism is generally associated with ophthalmological peculiarities such as nystagmus , strabismus , strong refractive errors, foveal dysgenesis, chorioretinal hypopigmentation. All mammals known to have an OCA1 defect have defects in the visual system even if only one OCA1 allele is present, which cannot be explained by the amount of melanin produced and the exact cause of which is still unknown. With one of these defects, a dysgenesis of the fovea centralis , there is no foveal depression, rather the retina in this area is as thick as anywhere else (300 µm compared to 150 µm). Since an estimated one to two percent of people heterozygous carry a mutation in TYR , it is believed that unexplained cases of stereo blindness may be due to it.
Individual evidence
- ↑ a b UniProt P14679 .
- ^ Skin / Hair / Eye pigmentation variation 3. In: Online Mendelian Inheritance in Man . (English).
- ↑ Isao Kubo, Ikuyo Kinst-Hori: "Tyrosinase inhibitory activity of the olive oil flavor compounds", in: Journal of Agricultural and Food Chemistry , 1999 , 47 (11) , pp. 4574-4578; doi : 10.1021 / jf990165v .
- ↑ Franz v. Bruchhausen, G. Dannhardt, Siegfried Ebel, August Wilhelm Frahm, Eberhard Hackenthal, Ulrike Holzgrabe: Hager's Handbook of Pharmaceutical Practice Volume 8: Substances EO . Springer-Verlag, 2013, ISBN 978-3-642-57994-3 , pp. 1032 ( limited preview in Google Book search).
- ↑ B. Käsmann-Kellner: "Albinism: Far more than just blue eyes", in: Ophthalmologe , 2007 , 104 (8) , pp. 646–647; doi : 10.1007 / s00347-007-1588-8 .
- ^ R. Morell, RA Spritz, L. Ho, J. Pierpont, W. Guo, TB Friedman, JH Asher Jr: Apparent digenic inheritance of Waardenburg syndrome type 2 (WS2) and autosomal recessive ocular albinism (AROA). , Human Molecular Genetics , Vol. 6, pp. 659-664.
- ↑ CH Meyer, DJ LaPolice, SF Freedman: foveal hypoplasia in oculocutaneous albinism demostrated by optical coherence tomography. In: American journal of ophthalmology. Volume 133, Number 3, March 2002, pp. 409-410, PMID 11860983 .
- ^ AG Leventhal, DJ Vitek, DJ Creel: Abnormal visual pathways in normally pigmented cats that are heterozygous for albinism. In: Science . Volume 229, Number 4720, September 1985, pp. 1395-1397, PMID 3929383 .
- ^ Tyrosinase. In: Online Mendelian Inheritance in Man . (English)