Unsaturated polyester resins
Unsaturated polyester resins ( UP resins ) are synthetic resins and are used to manufacture molded parts or composite materials . They are 60 to 70% solutions of unsaturated polyesters in a vinyl monomer, mostly styrene . Curing of the UP resins takes place via a free-radical chain polymerization , wherein a crosslinked copolymer of the polyester and the vinyl monomer forming and to a thermoset leads. UP resins are among the reactive resins , since no condensate is released during hardening.
Manufacturing
Linear polyester
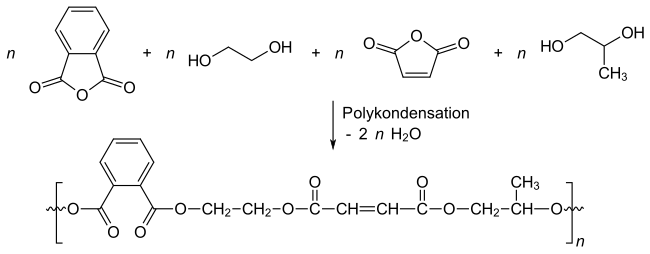
The polyesters are obtained by reacting diols and dicarboxylic acids or their anhydrides . The C = C double bonds are introduced by compounds such as fumaric acid , maleic acid , maleic anhydride , tetrahydrophthalic acid . Dicarboxylic acids or their anhydrides without a C = C double bond, such as phthalic anhydride , isophthalic acid or adipic acid, are used to regulate the unsaturated content in the product. Monomers such as 1,2-propanediol , ethylene glycol , diethylene glycol , 1,3-butanediol or 1,4-butanediol are used as diols .
The linear and uncrosslinked polyesters are produced by polycondensation at 150 to 200 ° C., the water which is split off being distilled off. The mean molar mass is typically 3000 to 4000 g / mol. The standard is the conversion of phthalic anhydride, ethylene glycol, 1,3-butanediol and maleic anhydride:
Vinyl monomer
The still warm polyesters are dissolved in 30 to 40% monomers with vinyl groups . The monomer used is mostly styrene , but also methyl methacrylate or diallyl phthalate . Additives such as accelerators for hardening or paraffins to inhibit the evaporation of the monomer during hardening can also be added to the more or less viscous product.
Hardening
| Peroxide initiators |
 Methyl ethyl ketone peroxide |
 Dibenzoyl peroxide |
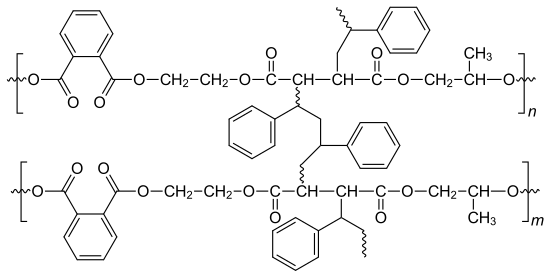
After adding 1 to 4% of a peroxide initiator, radical copolymerization begins between the vinyl monomer (here styrene) and the polyunsaturated macromolecules. Two to three styrene units form the links to neighboring macromolecules on the former double bonds.
Compounds such as dibenzoyl peroxide and methyl ethyl ketone peroxide are used as initiators . With the so-called warm curing, the polymerization only starts at 60 to 100 ° C. By adding accelerators such as cobalt salts of fatty acids and tertiary amines , the reaction starts at room temperature (cold hardening). The chain polymerization is of course exothermic even with cold curing. UV curing is also possible with photoinitiators . When hardening, there is a volume loss of 5–9%, which increases with the double bond content.
|
|
||||||||||||||
| Formation of radicals through reaction with tertiary amines. | Co 2+ / Co 3+ redox reactions act as a siccative . | |||||||||||||
properties
Without aggregates, the hardened resin is crystal clear, brittle and has good tracking resistance. Depending on the type, the application limit is between 100 and 185 ° C and the density between 1.17 and 1.26 g / cm 3 . Glass fibers are an important and common filler .
application
-
Laminates
- Vehicle industry: bodies, bodies of boats and sport aircraft
- Silos , tanks for heating oil and pipes for waste water
- Molding compounds
- Electronics: switch and plug parts, device housing
- Components for vehicles
-
Casting resins
- Embedding of electrical components, insulators
Individual evidence
- ↑ a b c d Bernd Tieke: Makromolekulare Chemie , 3rd edition, Wiley-VCH, Weinheim, 2014, p. 58 f.
- ↑ a b c Wolfgang Kaiser: Kunststoffchemie für Ingenieure , 3rd edition, Carl Hanser, Munich, 2011, p. 426 ff.
- ↑ Walter Hell Erich, Guenther Harsch, Erwin Baur: Material Guide plastics , 10th Edition, Carl Hanser, Munich, 2010, p 191. (Limited preview)