Chiller
A refrigeration machine uses a compressor to transport thermal energy from a colder place to be cooled to a warmer environment. Many refrigeration machines are based on a thermodynamic cycle . In a broader sense, the adsorption chillers and the absorption chillers are also included, although they do not have a mechanical drive (motor).
The purpose of a chiller is to cool it to a temperature below the ambient temperature.
Chillers are like heat pumps - the latter, however, uses the heat given off.
Chillers usually work according to the following principles:
- Cold vapor systems use evaporative cooling by means of refrigerants that have suitable evaporation temperatures for the desired temperature and pressure range. The refrigerant is constantly subjected to a phase transition from liquid to gas and vice versa.
- Machines that use the Joule-Thomson effect dispense with liquefaction and use the effect that gases cool down when they are expanded. See also Linde method . With multi-stage systems, very low temperatures, e.g. B. for air liquefaction can be achieved.
Cooling processes that do without gases and moving parts are not called refrigeration machines in the narrower sense. There are z. B. thermoelectric cooling ( Peltier effect ) and magnetic cooling ( magnetocaloric effect ).
history
The generation of cold by pumping out air (more precisely: pumping out of the gas phase (consisting of air and ether vapor) with the result of a pressure drop in the vessel and a decrease in the volume of the liquid) from a glass flask half filled with diethyl ether was discovered in the middle of the 18th century , but there was there are initially no practical applications.
The world's first functioning chiller was built in Florida in 1845 by the American doctor John Gorrie , who was looking for ways to improve the chances of recovery for hospital patients in hot, humid Florida. According to medical doctrines at the time, “bad air” was a major disease factor, and the winter ice brought in from the northern Great Lakes , which was the only way to cool, was very expensive in Florida because of the large transport losses. Gorrie's machine, which used the reverse principle of the Stirling engine, was used to make ice and at the same time to cool the room (air conditioning). A prototype was built. But the machine was a financial failure (patent application 8080, May 6, 1851). Gorrie died a few years later, impoverished and laughed at.
It was not until the 1870s that refrigeration machines became economical; the first major users were breweries , which were able to brew bottom-fermented , longer-lasting lager according to the Pilsen method, even without natural cool cave systems . The German industrialist Carl von Linde was one of the first major manufacturers .
Execution of the system for heat transfer
The cold "generated" by the refrigeration machine can be used for technical process purposes , for air conditioning , to make ice (ice rinks) or to preserve or cool food. The heat can be absorbed directly or indirectly. In the case of indirect cooling, a coolant (cold water or brine - often as a mixture with glycol to avoid freezing in the lines) is used, which is cooled in the first heat exchanger by the evaporating refrigerant and in the second heat exchanger absorbs the heat of the medium to be cooled . When used directly, a heat exchanger is used that carries the evaporating refrigerant on one side and the medium to be cooled on the other.
Types
The main difference between compression and sorption chillers is that in the former, the required energy is completely supplied as mechanical work, while in the latter it is supplied in the form of heat. The latter only require mechanical work to overcome the internal pressure losses.
The efficiency is usually based on compression refrigerating machine to the electrical drive energy, which arise as compared with sorption clearly more favorable values. A comparison of this type is not permitted, however, since mechanical or electrical drive energy is not available in nature, but has to be generated (converted) from fossil or regenerative sources with losses, which is also reflected in the energy price. If these losses are included, the efficiency of sorption chillers is also comparable in value, if not better.
The efficiency of refrigeration machines is called the coefficient of performance .
Absorption chillers
The absorption chiller also has a solvent and a refrigerant circuit. The working fluid consists of two components, a solvent and the refrigerant. The refrigerant must be completely soluble in the solvent. Absorption refrigeration machines with water as the refrigerant and an aqueous lithium bromide (LiBr) solution as the solvent are technically widespread. Evaporation temperatures of the water of up to approx. 3 ° C can be achieved through vacuum operation. Absorption chillers that use ammonia (NH 3 ) as the refrigerant and water as the solvent can reach lower temperatures . In large-scale ammonia absorption refrigeration systems, evaporation temperatures of −70 ° C are reached.
In the case of sorption chillers, the sorption heat, which has to be removed from the absorber or adsorber, is added as a further heating output .
Adsorption refrigeration systems
The adsorption chiller works with a solid solvent, the "adsorbent", on which the refrigerant is adsorbed or desorbed. Heat is added to the process during desorption and removed during adsorption. Since the adsorbent cannot be circulated in a cycle, the process can only run discontinuously. For this reason, two chambers with adsorbents are used, in which the adsorption and desorption takes place in parallel within one working cycle (6–10 minutes). After the end of the work cycle, heat supply and heat dissipation to the two chambers are exchanged (switchover, approx. 1 min.). Then the adsorption and desorption begin again in parallel. This means that almost even refrigeration can be guaranteed.
Diffusion absorption refrigerator
The diffusion absorption chiller works in a similar way to the absorption chiller, but the pressure change is implemented as a partial pressure change. This requires a third component for the working fluid, an inert gas . Their advantage is that the pressure hull is hermetically sealed and does not require any detachable seals, and that the apparatus works silently. The technology is used, for example, in camping and hotel refrigerators.
Compression refrigeration systems
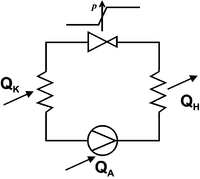
In the compression refrigeration machine , a working medium flows in a flow circuit, alternately absorbing heat at a lower temperature and releasing (more) heat at a higher temperature. Pumping, i.e. the introduction of mechanical work, is necessary to keep the flow and thus the process going. Such machines work either
- by alternating evaporation and condensation of the medium (refrigerant), or
- with an always gaseous medium (mostly air).
The first type is widespread and is used e.g. B. in household refrigerators , freezers and freezers, dispensing systems, cold storage, air conditioning , ice rinks, slaughterhouses, breweries and the chemical industry.
In the cycle, gaseous refrigerant is compressed by a compressor (using the drive energy ). In the downstream heat exchanger ( condenser ), the refrigerant condenses, whereby heat it at high temperature gives (condensation heat ) corresponding to the high also at the high pressure condensation temperature . The liquid refrigerant is directed to a throttle device, where its pressure is reduced. In the second heat exchanger ( evaporator ), the refrigerant then absorbs heat at low temperature by evaporation, e.g. B. from the cold room of a refrigerator (heat of vaporization ). The compressor sucks in the evaporated refrigerant again and the cycle is closed. It applies , where is the waste heat that the compressor gives off directly to the environment.
The second type also works with a compressor that compresses the working medium (often air), whereby it heats up. Then it gives off heat to the warm environment, where it cools down but remains gaseous. Then it flows z. B. by a turbine , which relaxes it and cools it down further. The turbine returns some of the mechanical work used by the compressor. The medium then absorbs heat from the room to be cooled before it is compressed again. The process can also be designed in an open way, by sucking in air from the environment, compressing it, cooling it and relaxing it, after which it is directed into the room to be cooled (e.g. the interior of a car or building).
Instead of a compressor and turbine, such a system can also work with a machine corresponding to the Schukey motor , which takes over the compression and expansion of the gas; this solution is technically simpler. This construction is of interest, among other things, for car air conditioning systems, where the advantage is particularly significant that no refrigerant is required that can escape in the event of an accident and may even pose a fire hazard (depending on the type of refrigerant).
The expansion with gain in mechanical work (turbine) can in principle also be replaced by a simple throttling of the gas, because real gas is cooled by adiabatic throttling (in contrast to ideal gas , whose temperature remains the same). The efficiency of the system is then much worse. The cooling of the gas is much less, and the usable work provided by the turbine is no longer required.
For the operation of a refrigeration machine, according to the second law of thermodynamics , energy must be supplied from the outside in the form of mechanical work, because only then can heat be transported from one place with a lower temperature to another with a higher temperature.
Steam jet cooling system
The steam jet refrigeration system is a thermal refrigeration system in which water vapor is used as a propellant, refrigerant and refrigerant. The expansion and relaxation of a jet of water vapor creates a vacuum and sucks in water vapor from an evaporator. The evaporation cools the water reservoir in the evaporator and can therefore be used as a coolant.
Joule-Thomson effect (JTE), Linde method
To generate cold, the temperature of a gas (e.g. air, helium) that does not condense out in the work area is reduced by throttling. With the JTE, a cooling of approx. 0.4 K per bar pressure difference (air approx. 1/4 K / bar, CO 2 approx. 3/4 K / bar) can be achieved at the throttle. Although this effect is apparently very small, it can also be used to achieve low temperatures close to absolute zero. Systems are often designed in several stages.
The apparatus representation of a Joule-Thomson system is similar to that of a compression refrigeration machine, but the heat exchangers are not built as a condenser or evaporator. For energetic optimization it is necessary to precool the gas in a recuperative (countercurrent) heat exchanger with the gas returning from the cooler upstream of the expansion valve (throttle).
In 1895, Carl von Linde used such a system for air liquefaction and liquefied quite large quantities (1 bucket / h) of air. The technical process for liquefying and separating air based on the Joule-Thomson effect has since been called the Linde process.
However, it is crucial for cooling according to the Joule-Thomson method that the starting temperature is below the inversion temperature T i of the respective gas. This is approx. +659 ° C for air, −80 ° C for hydrogen and −239 ° C for helium. If a gas is expanded below its inversion temperature, it cools down, if it is expanded above its inversion temperature, it heats up. In order to be able to cool a gas according to the JT process, the starting temperature must therefore be below the inversion temperature. For a van der Waals gas , this temperature can be calculated using the following equation, which corresponds to the internal pressure , the co-volume of the gas, the critical temperature and the universal gas constant .
Pulse tube cooler
A pulse tube cooler is a refrigeration machine whose functional principle corresponds roughly to a Stirling engine, but which does not require any mechanically moving parts. This makes very compact cooling heads possible and the minimum temperature that can be achieved is not limited by the mechanical frictional heat of these parts. The lowest temperature to date was reached around 1.3 K (= –272 ° C).
Thermoelectric effect, Peltier element
A Peltier element , which is operated electrically and does not require a refrigerant, can also be used for cooling (or heating) . With large temperature differences (50 ... 70 K), however, the cooling capacity drops to zero. For higher temperature differences, pyramid-shaped, multi-level structures are used.
This technology is used to stabilize the temperature of semiconductor lasers and sensors, in vehicle cool boxes, in thermal cyclers (PCR) and to cool image recorders in cameras from infrared to UV .
Magnetic cooling
Another cooling method is based on the magnetic properties of certain substances. When magnetized, some substances release heat; these are then called magnetocaloric substances. With magnetic cooling , the substance is brought into a magnetic field , whereby it heats up; the heat is mostly dissipated here by means of a cooling liquid. The substance, which has been brought back to ambient temperature, now leaves the magnetic field and demagnetizes itself in the area that is to be cooled. During demagnetization, the substance absorbs heat. Mechanical work must be applied from the outside in order to remove the magnetized material from the magnetic field.
Such cooling systems are usually more efficient than systems that work with steam, but more expensive because suitable magnetocaloric substances, e.g. B. gadolinium compounds are expensive.
Evaporative cooling
In evaporative cooling, energy in the form of heat ( enthalpy of evaporation ) is extracted from a medium (e.g. the air or a surface) through the evaporation of water . In the field of supply engineering, evaporative cooling is also often called adiabatic or adiabatic cooling, since the physical process is theoretically an isenthalpic conversion of sensible into latent heat . It is a heat transfer process from high to low temperature, enhanced by a phase transition (water to steam), and thus represents a self-running, "clockwise" (= cooling) thermodynamic cycle . Therefore, apart from the transport of air and water, no additional mechanical, electrical or thermal energy is required.
The possible extent of the cooling depends on the surrounding air temperature and humidity, i.e. relative humidity : With a relative humidity of the air close to 100%, i.e. air saturated or even oversaturated with water vapor (as in fog ), the effect is almost not noticeable , The saturation vapor pressure of the water in the air is too high. However, the lower the relative humidity, the higher the potential for further moisture absorption and the more water can thus evaporate and reduce the air temperature. All changes in the state of the air can be shown in the Mollier h, x diagram (absolute humidity versus temperature). The total energy content of the air is given in kJ / kg. Since the entire energy content does not change during evaporative cooling (adiabatic), the change of state always runs on the isenthalps in the diagram ( const kJ / kg) from top left to bottom right. The saturation line is finally reached at a relative humidity of 100%. For example, air with a temperature of 21 ° C and 40% relative humidity contains an enthalpy of 36.7 kJ / kg.If you follow the course of these isenthalps in the diagram, it intersects the dew point line at 13 ° C and 100% relative humidity. A lower temperature than 13 ° C cannot be reached here through evaporation.
Evaporative cooling represents the decisive physical process behind the cooling effect of sweating (or, for example, skin exposed to the wind and wetted by hand), the effectiveness of which, as discussed, e.g. B. is no longer given in a sauna. This type of cooling was also used early in the history of technology; since ancient times, clay vessels have been known that are moistened and allow evaporation via their open-pored surface, which cools their contents (e.g. butter cooler made of clay). Another example of early use is the wind tower in conjunction with a qanat . In terms of process technology, the effect is used in modern systems such. B. used in the wet cooling tower. In Central Europe, this can usually deliver a cooling water temperature of around 27 ° C at an outside temperature of 32 ° C, i.e. a temperature well below the ambient temperature.
Performance figure
The efficiency of chillers and heat pumps is given with the help of the coefficient of performance or performance figure. Depending on the context, the abbreviations EER ( English energy efficiency ratio ) for refrigeration machines and COP ( English coefficient of performance ) for heat pumps are used instead. These variables indicate the relationship between cooling or heating capacity and the technical (mechanical or electrical) capacity used.
For a refrigeration system that draws the cooling capacity from the cold reservoir using the technical capacity :
The technical output corresponds to the difference between the waste heat output and the consumed cooling output , so that:
- .
The efficiency of a heat engine is defined as the ratio of the technical power generated and the thermal power absorbed from the hot reservoir . It therefore corresponds to the reciprocal of the COP:
Statutory Regulations
For chillers there are legal regulations regarding the used refrigerants and efficiency. The EcoDesign ERP regulation for seasonal efficiency requirements is planned for 2021.
See also
literature
- IKET (Hrsg.): Pohlmann-Taschenbuch der Kältetechnik. Basics, applications, work tables and regulations . 19th revised and expanded edition 2008. CF Müller Verlag, Heidelberg 2008, ISBN 978-3-7880-7824-9
- Hans-Liudger Dienel : The place of research and development in German refrigeration engineering, 1880-1930 . In: Technikgeschichte, 62nd Vol. (1995), H. 1, pp. 49-69.
Individual evidence
- ↑ D. Lüdecke, C. Lüdecke: Thermodynamics . Springer-Verlag, 2000, ISBN 978-3-540-66805-3 , pp. 340 ( google.de [accessed December 27, 2013]).
- ↑ Chillers ready for EcoDesign ErP regulation 2021 .
Web links
- Oldest refrigeration machine in the world at Auer Mühlbach
- Information platform for refrigeration technology, basics, technology, publications, application of refrigeration technology, addresses, substances and regulations
- History of the refrigeration machine (PDF; 401 kB)
- Project information: Cold storage in large cold networks