Viola throne
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Viola throne | ||||||||||||||||||
| other names | |||||||||||||||||||
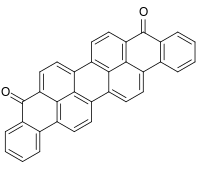
| Molecular formula | C 34 H 16 O 2 | ||||||||||||||||||
| Brief description |
blue-black to black powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 456.5 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
490-495 ° C (decomposition) |
||||||||||||||||||
| solubility |
in water <1000 mg l −1 at 19 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Violanthron ( CI Vat Blue 20 ) is a quinoid polycyclic aromatic hydrocarbon . The compound belongs to the carbonyl dyes and is used as a vat dye .
Manufacturing
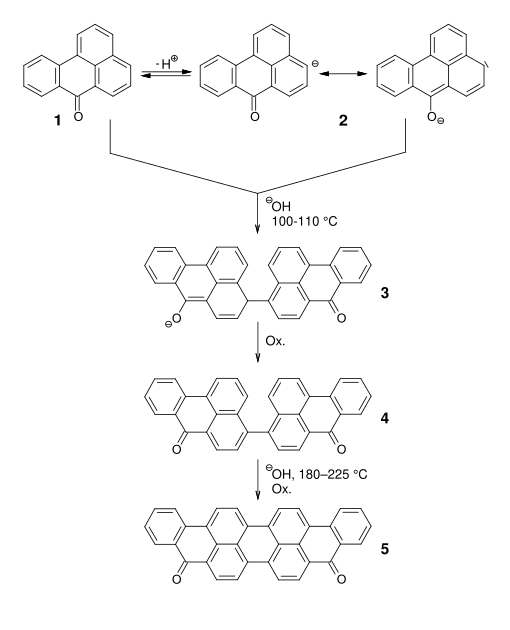
Starting from benzanthrone ( 1 ), a mesomeric-stabilized anion ( 2 ) is obtained under basic conditions , which dimerizes spontaneously in the presence of an oxidizing agent . The dimerization product ( 4 ) can cyclize under alkaline conditions and a temperature of 180-225 ° C. to form violanthrone ( 5 ) .
Black vat dyes are obtained by nitrating Violanthron, although the exact number and substitution sites of the nitro groups is not known. The nitro compound can be reduced to the corresponding amine derivative , the dye CI Vat Green 9.
Web links
Individual evidence
- ↑ a b c Chemical Datasheet Vat Blue 20. Cameo Chemicals, accessed March 20, 2019 .
- ↑ a b SAFETY DATA SHEET (A193981). Ambeed, Inc., accessed March 20, 2019 .
- ^ A b Heinrich Zollinger: Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments . 3. Edition. WILEY-VCH Verlag, Weinheim 2003, ISBN 3-906390-23-3 , p. 291 ff . ( limited preview in Google Book search).