Benzanthrone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
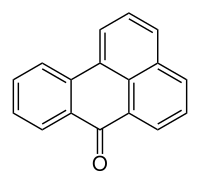
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Benzanthrone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 17 H 10 O | ||||||||||||||||||
| Brief description |
yellow-orange powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 220.27 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
170 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
0.15 g m −3 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Benzanthrone is a polycyclic aromatic compound belonging to the class of ketones . The compound is a preliminary product for the manufacture of vat dyes .
presentation
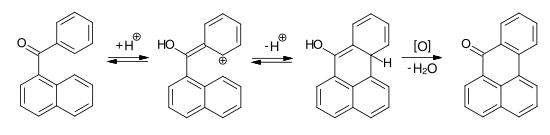
An important reaction for the preparation of fused anthraquinone dyes is the Scholl reaction . Starting from 1-naphthylphenyl ketone with AlCl 3 and traces of water, after protonation of the starting compound and subsequent intramolecular electrophilic aromatic substitution, an enol intermediate is obtained, which is then oxidized to benzanthrone.
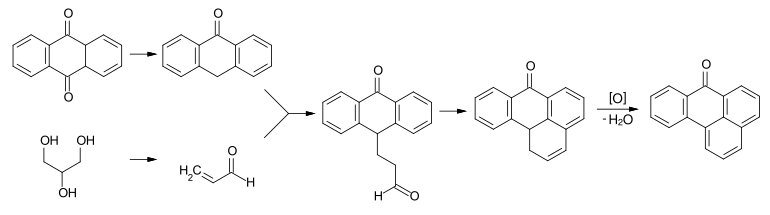
The industrial production of benzanthrone is based on anthraquinone . As in the Skraup synthesis, anthraquinone and glycerine are converted in concentrated sulfuric acid and in the presence of elemental iron . Under these conditions, anthraquinone is reduced to anthracen-9 (10``H '') - one and glycerol is dehydrated to acrolein . Acrolein reacts with the reduced anthraquinone to form an aldehyde intermediate , which cyclizes intramolecularly in the sense of a Friedel-Crafts alkylation . The resulting dihydrobenzanthrone is then finally oxidized to benzanthrone.
Benzanthrone is the starting compound for various vat dyes from the class of carbonyl dyes , for example violanthrone .
Web links
Individual evidence
- ↑ a b c d e Entry on benzanthrone in the GESTIS substance database of the IFA , accessed on March 21, 2019(JavaScript required) .
- ^ Heinrich Zollinger: Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments . 3. Edition. WILEY-VCH Verlag, Weinheim 2003, ISBN 3-906390-23-3 , p. 286 f . ( limited preview in Google Book search).