Tungsten (VI) oxide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ W 6+ __ O 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Tungsten (VI) oxide | |||||||||||||||
| other names |
Tungsten trioxide |
|||||||||||||||
| Ratio formula | WHERE 3 | |||||||||||||||
| Brief description |
lemon yellow powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 231.85 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
7.16 g cm −3 |
|||||||||||||||
| Melting point |
1473 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tungsten (VI) oxide , also tungsten trioxide (WO 3 ), is the most important oxide of tungsten .
Occurrence
Of course, tungsten (VI) oxide only occurs with crystal water in the form of the minerals elsmoreit (tungsten (VI) oxide hemihydrate), tungstite (tungsten (VI) oxide hydrate) and meymacite (tungsten (VI) oxide dihydrate).
Manufacturing
The extraction of tungsten trioxide takes place by annealing tungsten or tungsten compounds with access to air .
It can also be prepared by reacting sodium tungstate dihydrate with hydrochloric acid .
properties
Tungsten (VI) oxide is an intensely yellow colored crystal powder at room temperature and orange colored when heated. Tungsten trioxide is completely insoluble in water and acids, but can react with water to form tungstic acid. It reacts with alkalis to form tungstates .
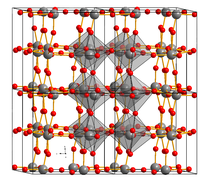
The crystal structure of WO 3 is made of WO 6 - octahedra which are connected in the three spatial directions shared corners each other. As can be seen in the figure, these octahedra can be tilted against each other. As a result, three polymorphs , ie different structures, appear even below room temperature . It only remains at low symmetry and changes from monoclinic via triclinic back to monoclinic. Only at high temperatures does it change into an orthorhombic and a tetragonal phase. The position of the oxide ions in particular differs slightly.
use
Tungsten trioxide is used in the ceramic industry as a contact and as a yellow pigment . WO 3 could acquire a certain importance in the production of ultra-thin oxide films with which optical lenses can be coated so as to be scratch-resistant. Also electrochromic glazing containing tungsten trioxide.
Individual evidence
- ↑ a b c Entry on tungsten oxides. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b Entry on tungsten trioxide in the GESTIS substance database of the IFA , accessed on November 11, 2007(JavaScript required) .
- ↑ a b Tungsten (VI) oxide data sheet from Sigma-Aldrich , accessed on April 25, 2011 ( PDF ).
- ^ Mineralienatlas: Tungstit
- ↑ Georg Brauer (ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1566.
- ^ HA Wriedt: The OW (oxygen-tungsten) system. In: Bulletin of Alloy Phase Diagrams. 10, 1989, p. 368, doi : 10.1007 / BF02877593 .
- ^ W. Kleber, M. Hahnert, R. Müller: WO3 - his cultivation and crystallographic investigation. In: Journal of Inorganic and General Chemistry. 346, 1966, p. 113, doi : 10.1002 / zaac.19663460302 .
Web links
- Peter Pietschmann: Optical filters. Ulm University, press release of October 23, 1997 from the Science Information Service (idw-online.de), accessed on August 24, 2015.


