Moniliformis: Difference between revisions
adding note |
→Taxonomy and description: separating |
||
| (32 intermediate revisions by 13 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Genus of worms}} |
|||
{{Automatic taxobox |
{{Automatic taxobox |
||
| taxon = Moniliformis |
|||
| image = Moniliformis moniliformis.jpg |
| image = Moniliformis moniliformis.jpg |
||
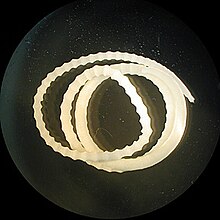
| image_caption = Adult ''[[Moniliformis moniliformis]]'' |
| image_caption = Adult ''[[Moniliformis moniliformis]]'' |
||
| taxon = Moniliformis |
|||
| authority = Travassos, 1915 |
| authority = Travassos, 1915 |
||
}} |
}} |
||
'''Moniliformis''' is a |
'''''Moniliformis''''' is a genus of parasitic worms in the [[Acanthocephala]] phylum. |
||
==Taxonomy== |
|||
Genetic analysis has been conducted on four species: ''Moniliformis moniliformis'', ''M. saudi'', ''M. cryptosaudi'' and ''M. kalahariensis''.<ref name="AminHeckmann2016"/><ref name="Amin2019"/> Based on these results, Moniliformidae has been determined to be [[monophyletic]].<ref name="AminHeckmann2016"/><ref name="Amin2019"/> |
|||
{{cladogram|align=center|title=Archiacanthocephala |
|||
|caption=Phylogenetic reconstruction for select species in the class Archiacanthocephala<ref name="Gomes 2019">{{cite journal |last1=Nascimento Gomes |first1=Ana Paula |last2=Cesário |first2=Clarice Silva |last3=Olifiers |first3=Natalie |last4=de Cassia Bianchi |first4=Rita |last5=Maldonado |first5=Arnaldo |last6=Vilela |first6=Roberto do Val |title=New morphological and genetic data of Gigantorhynchus echinodiscus (Diesing, 1851) (Acanthocephala: Archiacanthocephala) in the giant anteater Myrmecophaga tridactyla Linnaeus, 1758 (Pilosa: Myrmecophagidae) |journal=International Journal for Parasitology: Parasites and Wildlife |date=December 2019 |volume=10 |pages=281–288 |doi=10.1016/j.ijppaw.2019.09.008|pmid=31867208 |pmc=6906829}}</ref><ref name="Amin2020">{{Cite journal |doi = 10.1017/S0022149X20000073|title = New perspectives on Nephridiacanthus major (Acanthocephala: Oligacanthorhynchidae) collected from hedgehogs in Iran|year = 2020|last1 = Amin|first1 = O.M.|last2 = Sharifdini|first2 = M.|last3 = Heckmann|first3 = R.A.|last4 = Zarean|first4 = M.|journal = Journal of Helminthology|volume = 94|pages = e133|pmid = 32114988|s2cid = 211725160}}</ref> |
|||
|cladogram= |
|||
{{clade |
|||
|label1=[[Archiacanthocephala]] |
|||
|1={{clade |
|||
|label1=[[Oligacanthorhynchidae]] |
|||
|1= {{clade |
|||
|1=''[[Macracanthorhynchus ingens]]'' |
|||
|2=''[[Oncicola venezuelensis]]'' |
|||
|3=''[[Oligacanthorhynchus tortuosa]]'' |
|||
|4=''[[Nephridiacanthus major]]''}} |
|||
|2={{clade |
|||
|1=''[[Moniliformis moniliformis]]'' |
|||
|label1='''Moniliformidae''' |
|||
|label2=[[Gigantorhynchida]] |
|||
|2={{clade |
|||
|1=''[[Mediorhynchus]]'' sp. |
|||
|2=''[[Gigantorhynchus echinodiscus]]'' |
|||
}} |
|||
}} |
|||
}} |
|||
}} |
|||
}} |
|||
==Description== |
|||
Species of the genus Moniliformis are usually pseudosegmented and have a cylindrical proboscis with longitudinal rows of hooks that have posteriorly directed roots. Moniliformis are further characterized by the presence of a simple, double-walled proboscis receptacle with the outer wall having spirally aligned muscle fibers, brain at posterior end of receptacle, and dorsal and ventral lacunar canals.<ref name="Schmidt1989">{{cite journal |doi= 10.2307/3282769|pmid = 2926590|jstor = 3282769|title = ''Australiformis semoni'' (Linstow, 1898) n. Gen., n. Comb. (Acanthocephala: Moniliformidae) from Marsupials of Australia and New Guinea|journal = The Journal of Parasitology|volume = 75|issue = 2|pages = 215–7|year = 1989|last1 = Schmidt|first1 = Gerald D.|last2 = Edmonds|first2 = Stanley J.}}</ref><ref name="Kükenthal">{{cite book |last=Kükenthal|first=W |date=2014 |title=Gastrotricha and Gnathifera |url=https://books.google.com/books?id=hwCTBgAAQBAJ&q=apororhynchus&pg=PP11 |location=[[Göttingen]], Germany |publisher=[[Walter de Gruyter]] |page=322 |isbn=978-3110274271}}</ref> The proboscis retractor muscles pierce both the posterior and ventral end or just posterior end of the receptacle.<ref name="Amin1987">{{cite journal |last1=Amin |first1=O. M.|date=1987 |title=Key to the families and subfamilies of Acanthocephala, with the erection of a new class (Polyacanthocephala) and a new order (Polyacanthorhynchida) |journal=Journal of Parasitology |volume=73 |issue=6 |pages=1216–1219 |doi=10.2307/3282307 |jstor=3282307 |pmid=3437357}}</ref> The cerebral ganglion is in the mid to posterior region, and the lemnisci are long and flat and not bound to the body wall. These worms also lack [[protonephridia]] and males have eight cement glands, each with a giant nucleus, which are used to temporarily close the posterior end of the female after copulation.<ref name="Schmidt1989" /><ref name="Kükenthal" /><ref name="Bush2001">{{cite book | last1=Bush | first1=Albert O.| last2=Fernández |first2=Jacqueline C.| last3=Esch| first3=Gerald W.| last4=Seed| first4=J. Richard | title=Parasitism : the diversity and ecology of animal parasites | publisher=Cambridge University Press | location=Cambridge, UK New York, NY | year=2001 | isbn=0-521-66278-8 | oclc=44131774 | page=203}}</ref> |
|||
==Species== |
|||
{|class="wikitable floatright" style="text-align: center; font-size: 75%;" |
|||
! colspan="3" |''M. acomysi'' |
|||
|- |
|||
! Measurements<ref name="Martins2017"/>||Female (mm)||Male (mm) |
|||
|- |
|||
|Length of [[proboscis]]||0.20–0.43||0.19–0.36 |
|||
|- |
|||
|Width of [[proboscis]]||0.14–0.22||0.12–0.21 |
|||
|- |
|||
|Length of [[proboscis]] receptacle ||0.68–0.84||0.68–0.84 |
|||
|- |
|||
|Width of [[proboscis]] receptacle ||0.24–0.33||0.24–0.33 |
|||
|- |
|||
|Length of trunk||22–89||12–76 |
|||
|- |
|||
|Width of trunk||0.56–1.50||0.26–1.50 |
|||
|- |
|||
|Size of [[lemniscus (anatomy)|lemnisci]]||2.73–4.42 × 0.13–0.23||2.73–4.42 × 0.13–0.23 |
|||
|- |
|||
|Size of testis||colspan="2" |2.31 × 0.52–0.65 |
|||
|- |
|||
|Size of cement glands||colspan="2" |0.300 |
|||
|- |
|||
|Size of eggs||colspan="2" |0.07–0.09 x 0.03–0.05 |
|||
|- |
|||
|} |
|||
{|class="wikitable floatright" style="text-align: center; font-size: 75%;" |
|||
! colspan="3" |''M. aegyptiacus'' |
|||
|- |
|||
! Measurements<ref name="Martins2017"/>||Female (mm) |
|||
|- |
|||
|Length of [[proboscis]]||0.35 |
|||
|- |
|||
|Width of [[proboscis]]||0.16–0.18 |
|||
|- |
|||
|Length of [[proboscis]] receptacle ||0.75 |
|||
|- |
|||
|Length of trunk||115 |
|||
|- |
|||
|Length of [[lemniscus (anatomy)|lemnisci]]||5.25–6.0 |
|||
|- |
|||
|Size of eggs||colspan="2" |0.09–0.1 x 0.04–0.05 |
|||
|- |
|||
|} |
|||
{|class="wikitable floatright" style="text-align: center; font-size: 75%;" |
|||
! colspan="3" |''M. amini'' |
|||
|- |
|||
! Measurements<ref name="Martins2017"/>||Female (mm)||Male (mm) |
|||
|- |
|||
|Length of [[proboscis]]||0.28–0.30||0.27 |
|||
|- |
|||
|Width of [[proboscis]]||0.10–0.11||0.09 |
|||
|- |
|||
|Length of [[proboscis]] receptacle ||0.54–0.64||0.40 |
|||
|- |
|||
|Width of [[proboscis]] receptacle ||0.18–0.25||0.17 |
|||
|- |
|||
|Length of trunk||63.96–120.83||20.21 |
|||
|- |
|||
|Width of trunk||0.90–1.22||0.82 |
|||
|- |
|||
|Length of [[lemniscus (anatomy)|lemnisci]]||6.90–11.90||5.40 |
|||
|- |
|||
|Size of anterior testis||colspan="2" |1.80 × 0.60 |
|||
|- |
|||
|Size of posterior testis||colspan="2" |1.80 × 0.50 |
|||
|- |
|||
|Size of region occupied by cement glands||colspan="2" |0.40 × 0.17 |
|||
|- |
|||
|Size of eggs||colspan="2" |0.06 x 0.02 |
|||
|- |
|||
|Length of uterine bell||colspan="2" |1.17 |
|||
|- |
|||
|Distance from the [[uterine bell]] to genital pore||colspan="2" |1.08 |
|||
|- |
|||
|} |
|||
The genus ''Moniliformis'' <small>Travassos, 1915</small> contains eighteen species.<ref name="Amin2013">{{cite journal |last1=Amin |first1=Omar M. |title=Classification of the Acanthocephala |journal=Folia Parasitologica |date=September 19, 2013 |volume=60 |issue=4 |pages=273–305 |doi=10.14411/fp.2013.031 |pmid=24261131 |doi-access=free}}</ref><ref name="ITIS1915">{{cite web |url=https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=196573#null|title=Moniliformis Travassos, 1915|date= November 3, 2023 |website=Integrated Taxonomic Information System (ITIS) |access-date= November 3, 2023}}</ref>{{#tag:ref|A [[Binomial nomenclature|binomial authority]] in parentheses indicates that the species was originally described in a genus other than the present genus.|group=lower-alpha}} Description of the genus is the same as the family Moniliformidae with the exception of possessing spiral muscles in the outer wall of the proboscis receptacle<ref name="Schmidt1989"/> and a single distinct kind of proboscis hooks.<ref name="Ward1967"/> |
|||
*'''''Moniliformis acomysi''''' <small>Ward and Nelson, 1967</small><ref name="Ward1967">{{Cite journal |doi = 10.2307/3276638|jstor = 3276638|title = Acanthocephala of the Genus Moniliformis from Rodents of Egypt with the Description of a New Species from the Egyptian Spiny Mouse (''Acomys cahirinus'')|journal = The Journal of Parasitology|volume = 53|issue = 1|pages = 150–156|year = 1967|last1 = Ward|first1 = Helen L.|last2 = Nelson|first2 = Diane R.|pmid = 6066757}}</ref> |
|||
''M. acomysi'' was found infesting the [[Cairo spiny mouse]] (''Acomys cahirinus'') in [[South Sinai]], [[Egypt]].<ref name="Ward1967"/> The proboscis has eleven to sixteen longitudinal rows of hooks with six to ten hooks per row with the largest hooks being apical and range from 21 to 29 mm long in the male and 23 to 31 mm long in the female. There are eight cement glands in the male and the eggs of the female are oval.<ref name="Martins2017"/> The species name ''acomysi'' derives from the host genus name ''Acomys''.<ref name="Ward1967"/> |
|||
*'''''Moniliformis aegyptiacus''''' <small>Meyer, 1932</small><ref name="Meyer1932">{{cite journal |last1=Meyer |first1=A. |date=1932 |title= Acanthocephala|journal=Dr. H.G. Bronn's Klassen und Ordnungen des TierReichs |language=de|publisher=Akad. Verlag, Leipzig|volume=4 |pages=1–332}}</ref> |
|||
''M. aegyptiacus'' infests ''[[Meriones sinaiticus]]'' and the [[four-toed jerboa]] (''Allactaga tetradactyla'' reported as ''Scarturus tetradactylus'') in [[Egypt]] and the [[North African hedgehog]] (''Atelerix algirus'' reported as ''Erinaceus algiris'') in [[Tunis]]. The proboscis has 96 hooks arranged in twelve longitudinal rows with eight hooks per row. The eggs are oval.<ref name="Martins2017"/> The species name ''aegyptiacus'' derives from locality of the host, Egypt.<ref name="Meyer1932"/> |
|||
*'''''Moniliformis amini''''' <small>Martins, del Rosario Robles, and Navone 2017</small><ref name="Martins2017">{{cite journal |last1=Guerreiro Martins |first1=Natalia Beatriz |last2=del Rosario Robles |first2=María |last3=Navone |first3=Graciela Teresa |title=A new species of Moniliformis from a Sigmodontinae rodent in Patagonia (Argentina) |journal=Parasitology Research |date=August 2017 |volume=116 |issue=8 |pages=2091–2099 |doi=10.1007/s00436-017-5508-9 |pmid=28585077 |s2cid=33203157}}</ref> |
|||
''M. amini'' was found in the small intestine parasitising the [[olive grass mouse]] (''Abrothrix olivaceus'') in the [[Santa Cruz Province, Argentina]]. The species name ''amini'' was named after Omar Amin, a parasitologist specializing in Acanthocephala. [[Sexual dimorphism]] is pronounced with female over four times longer than the male. The proboscis has 12 to 14 longitudinal rows of hooks with 10 to 12 hooks per row with the largest hooks in the female being apical ranging from 22.21 to 24.47 μm, the middle being 15.50 μm long and the posterior being the smallest at 12 μm long. The largest hooks in the male are 24 μm. There are eight cement glands in the male, the eggs are oval.<ref name="Martins2017"/> |
|||
*'''''Moniliformis cestodiformis''''' <small>([[Otto Friedrich Bernhard von Linstow|Linstow]], 1904)</small>{{#tag:ref|''M. cestodiformis'' originally named ''Echinorhynchus cestodiformis'' by von Linstow in 1904<ref name="Linstow1904">{{cite journal |last1= von Linstow|first1=O. |date=1904 |title=Neue Helminthen aus Westafrika |journal=Centralbl. Bakt. Parasitenk., U. Lnfektionskr., Abt |volume=1 |issue=36 |pages=380–383}}</ref> but was renamed by Travassos in 1917. It is [[Synonym (taxonomy)|synonymous]] with ''Moniliformis erinacei'' <small>Southwell and Macfie, 1925</small><ref name="ITIS">{{cite web |url=https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=196572|title=Moniliformida Schmidt, 1972|date=November 23, 2019 |website=Integrated Taxonomic Information System (ITIS) |access-date=January 30, 2020}}</ref><ref name="AminHeckmann2016"/>|group=lower-alpha}} |
|||
''M. cestodiformis'' has been found infesting hedgehogs of the genus ''[[Erinaceus]]'' in [[West Africa]]. The proboscis is between 400 and 500 µm long and 200 µm wide with 16 to 18 rows of 7 or 8 hooks each and no apical pores. The largest hooks are found anteriorly and are 30 to 32 µm long with no dorsoventral differences.<ref name="AminHeckmann2016"/> |
|||
The eggs have 4 membranes and are 85 to 92 µm long and 49 to 51 µm wide giving them an elongation ratio of 1.73.<ref name="AminHeckmann2016"/><ref name ="eggs">{{cite thesis |last1=Pfenning |first1=Alana |title=Egg morphology, dispersal, and transmission in acanthocephalan parasites: integrating phylogenetic and ecological approaches |date=20 August 2017 |url=https://via.library.depaul.edu/csh_etd/272/}}</ref> |
|||
*'''''Moniliformis clarki''''' <small>(Ward, 1917)</small>{{#tag:ref|''M. clarki'' was originally named ''Hormorhynchus clarki'' by Ward in 1917 but was renamed by Chandler in 1921. It is [[Synonym (taxonomy)|synonymous]] with ''M. spiradentatis'' <small>Mcleod, 1933</small>.<ref name="ITIS"/><ref name="AminHeckmann2016"/>|group=lower-alpha}} |
|||
{|class="wikitable floatright" style="text-align: center; font-size: 75%;" |
|||
! colspan="3" |''M. Clarki'' |
|||
|- |
|||
! Measurements<ref name="Martins2017"/>||Female (mm)||Male (mm) |
|||
|- |
|||
|Length of [[proboscis]]||?||0.41–0.52 |
|||
|- |
|||
|Width of [[proboscis]]||?||0.13–0.14 |
|||
|- |
|||
|Length of [[proboscis]] receptacle ||?||0.65–0.67 |
|||
|- |
|||
|Width of [[proboscis]] receptacle ||?||0.32–0.33 |
|||
|- |
|||
|Length of trunk||120–250||61–85 |
|||
|- |
|||
|Width of trunk||2.10–2.80||1.60–2.00 |
|||
|- |
|||
|Length of [[lemniscus (anatomy)|lemnisci]]||?||3.46–4.79 × 0.13–0.18 |
|||
|- |
|||
|Size of anterior testis||colspan="2" |3.30–5.62 × 0.72–1.15 |
|||
|- |
|||
|Size of posterior testis||colspan="2" |3.97–4.29 × 0.72–1.17 |
|||
|- |
|||
|Size of eggs||colspan="2" |0.05–0.09 x 0.03–0.0 |
|||
|- |
|||
|} |
|||
''M. clarki'' commonly infects squirrels, chipmunks, deer mice, and gophers in North America.<ref name="AminHeckmann2016"/> If left untreated, it can kill its host. Ten [[cotton-top tamarins]] (''Saguinus oedipus'') were infected at the [[St. Louis Zoo]] with at least one being killed by the [[Parasitism|parasite]].<ref name="Weber2000">{{cite journal |title=Identification and treatment of Moniliformis clarki (Acanthocephala) in cotton-topped tamarins (''Saguinus oedipus''). |journal=Journal of Zoo and Wildlife Medicine |volume=31 |issue=4 |pages=503–7 |pmid=11428397 |year=2000 |last1=Weber |first1=M. |last2=Junge |first2=R. |doi=10.1638/1042-7260(2000)031[0503:IATOMC]2.0.CO;2 |s2cid=44668380}}</ref> Eggs of what is thought to be ''M. clarki'' due to the heavy population of common rodent hosts were discovered while examining human [[coprolite]] specimens gathered from [[Danger Cave]] in [[Utah]]. It was theorized that the man had either eaten a raw insect or an under cooked rodent.<ref name="Moore1969">{{Cite journal|title=Thomy-headed worm infection in north american prehistoric man.|journal = Science|volume = 163|issue = 3873|pages = 1324–5|pmid = 17807812|year = 1969|last1 = Moore|first1 = J. G.|last2 = Fry|first2 = G. F.|last3 = Englert Jr|first3 = E.|doi = 10.1126/science.163.3873.1324|bibcode = 1969Sci...163.1324M|s2cid = 6120428}}</ref> The female is twice as long than the male. The proboscis has 12 or 13 longitudinal rows of hooks with six or seven hooks per row with the largest hooks being 23 to 28 mm long.<ref name="Martins2017"/> The eggs have 4 membranes and are 75 μm long and have an elongation ratio of 2.42.<ref name ="eggs"/> |
|||
*'''''Moniliformis convolutus''''' <small>Meyer, 1932</small> |
|||
''M. convolutus'' has been found infesting the [[large flying fox]] (''Pteropus vampyrus'') in [[Brazil]]. The proboscis is 500 µm long by 200 µm wide with 12 rows of 11 or 12 hooks each. The posterior hooks are rootless and the proboscis receptacle is 700 µm long.<ref name="AminHeckmann2016"/> |
|||
*'''''Moniliformis cryptosaudi''''' <small>Amin, Heckmann, Sharifdini, and Albayati, 2019</small><ref name="Amin2019">{{Cite journal | doi=10.2478/s11686-018-00021-9| pmid=30666546| title=''Moniliformis cryptosaudi'' n. sp. (Acanthocephala: Moniliformidae) from the Long-eared Hedgehog ''Hemiechinus auritus'' (Gmelin) (Erinaceidae) in Iraq; A Case of Incipient Cryptic Speciation Related to ''M. saudi'' in Saudi Arabia| journal=Acta Parasitologica| volume=64| issue=1| pages=195–204| year=2019| last1=Amin| first1=Omar M.| last2=Heckmann| first2=Richard A.| last3=Sharifdini| first3=Meysam| last4=Albayati| first4=Nagham Yaseen |s2cid=58572640 | doi-access=free}}</ref> |
|||
''M. cryptosaudi'' is a [[Species complex#Cryptic species|cryptic species]] with ''M. saudi'' being morphologically identical, apart from the metal content of the hooks, but genetically distinct. The hooks consist of a [[collagen]] material with very low levels of [[phosphorus]] and [[calcium]] which is in contrast to those of ''M. saudi'' which has high levels of these two metals. The trunk is between 20.50 ad 48.75 mm long by 0.72 to 1.05 mm wide in the male and 28.75 to 121.25 mm long by 0.65 to 2.25 mm wide in the much larger female.<ref name="Amin2019"/> The anterior trunk tapers in a fan-shaped pattern and has a collar of vertical plates.<ref name="AminHeckmann2016"/> The proboscis is between 343 and 416 µm long by 135 and 188 µm wide in the male and 300 to 416 µm long by 162 to 291 µm wide in the female. The proboscis is armed with 13 to 14 rows of hooks each containing 7 or 8 hooks with the largest being found anteriorly and measuring 25 to 35 µm long in the female and 16 to 25 µm long in the male. The ventral hooks longer than dorsal hooks. The proboscis receptacle is 624 to 1092 µm by 218 to 364 µm in the female and 551 to 936 µm by 200 to 291 µm in the male. The long lemnisci has 6 to 8 giant nuclei and measures 4.26 to 7.28 mm by 0.12 to 0.18 mm in the male and has 6 to 9 giant nuclei and measures 4.15 to 8.12 mm by 0.11 to 0.21 mm in the female. The short lemnisci has 3 or 4 giant nuclei and measures 3.74 to 5.72 mm by 0.09 to 0.15 mm in the male and has 5 or 6 giant nuclei and measures 2.18 to 7.25 mm by 0.11 to 0.21 mm in the female. The anterior testes are 1.65 to 3.67 mm by 0.40 to 0.62 mm in size and the posterior testes are 1.50 to 2.62 mm by 0.37 to 0.62 mm in size. In the male, the cement glands are 322 to 780 µm by 229 to 416 µm and [[Saefftigen's pouch]] measures 624 to 925 µm by 270 to 425 µm. In the female, The eggs are oval, measuring 50 to 104 µm by 25 to 36 µm.<ref name="Amin2019"/> |
|||
The species name ''cryptosaudi'' refers to this cryptic status with ''M. saudi''. It has been found in the intestine infesting the [[long-eared Hedgehog]] (''Hemiechinus auritus'') near [[Baqubah]], [[Iraq]].<ref name="Amin2019"/> |
|||
*'''''Moniliformis echinosorexi''''' <small>Deveaux, Schmidt and Krishnasamy, 1988</small><ref name="Deveaux1988">{{Cite journal |doi = 10.2307/3282462|pmid = 3128654|jstor = 3282462|title = Two New Species of Moniliformis (Acanthocephala: Moniliformidae) from Malaysia|journal = The Journal of Parasitology|volume = 74|issue = 2|pages = 322–5|year = 1988|last1 = Deveaux|first1 = Timothy P.|last2 = Schmidt|first2 = Gerald D.|last3 = Krishnasamy|first3 = M.}}</ref> |
|||
''M. echinosorexi'' has been found infesting the [[moonrat]] (''Echinosorex gymnurus'') in [[Malaysia]] but may also infect hedgehogs. The worms have 12 to 15 rows of between 11 and 13 hooks that change from 34 to 36 μm long. The proboscis receptacle is 1.2 to 2.0 mm long.<ref name="Deveaux1988"/> Males are between 95 and 98 mm long whereas females are longer ranging from 126 to 192 mm long. The proboscis is relatively large ranging from 690 to 1000 µm long with 12 to 15 rows of containing 11 and 13 hooks each. The posterior hooks do not have roots. The proboscis receptacle is large at 1.2 mm long in males and 2.0 mm long in females. The testes are relatively large being between 5.6 and 6.8 mm long. Lemnisci are similarly long at with three giant nuclei each.<ref name="AminHeckmann2016"/> The species name ''echinosorexi'' derives from the genus of the host, ''Echinosorex''.<ref name="Deveaux1988"/> |
|||
*'''''Moniliformis gracilis''''' <small>(Rudolphi, 1819)</small>{{#tag:ref|''M. gracilis'' was originally named '' Echinorhynchus gracilis'' by Rudolphi in 1819 but was renamed by Meyer in 1931.<ref name="ITIS"/><ref name="Amin2013" />|group=lower-alpha}} |
|||
''M. gracilis'' is a parasite of the [[European roller]] (''Coracias garrullus'') in [[Europe]]. The worms are very small being only 2.25 to 3.00 mm long. The proboscis has 10 rows each with 8 or 9 hooks with the largest hooks on the anterior portion. The ganglion at the base of the short receptacle is 360 µm long.<ref name="AminHeckmann2016"/> |
|||
*'''''Moniliformis ibunami'''''<ref>{{cite journal |last1=Lynggaard |first1=Christina |last2=García-Prieto |first2=Luis |last3=Guzmán-Cornejo |first3=Carmen |last4=García-Varela |first4=Martín |title=Description of a new species of Moniliformis (Acanthocephala: Moniliformidae) from Peromyscus hylocetes (Rodentia: Cricetidae) in Mexico |journal=Parasitology International |date=1 August 2021 |volume=83 |pages=102315 |doi=10.1016/j.parint.2021.102315 |pmid=33677125 |s2cid=232142438}}</ref> |
|||
*'''''Moniliformis kalahariensis''''' <small>Meyer, 1931</small> |
|||
''M. kalahariensis'' was found infesting the [[Southern African Hedgehog]] (''Atelerix frontalis'') in [[Mohlonong]] and at the [[University of Limpopo]] in the [[Limpopo Province]], [[South Africa]].<ref name="Halajian2018">{{Cite journal |doi = 10.3897/zookeys.789.27710|pmid = 30344432|pmc = 6193052|issue = 789|pages = 1–18|title = Checklist of acanthocephalan parasites of South Africa|journal = ZooKeys|year = 2018|last1 = Halajian|first1 = Ali|last2 = Warner|first2 = Lesley R.|last3 = Tavakol|first3 = Sareh|last4 = Smit|first4 = Nico J.|last5 = Luus-Powell|first5 = Wilmien J.| bibcode=2018ZooK..789....1H |doi-access = free}}</ref> and [[Namaqua sandgrouse]] (''Pterocles namaqua'') in [[Botswana]]. The [[Host (biology)#Types of hosts|intermediate host]] is the [[German cockroach]] (''Blattella germanica'').<ref name="Amin2014"/> |
|||
Juvelines are unsegmented whereas adults are pseudosegmented. There are 2 apical sensory pores on the proboscis. Males trunk is between 44.25 and 75.00 mm long by 1.62 and 1.75 mm wide whereas the females are larger being 51.25 to 81.25 mm long by 2.00 to 2.25 mm wide. In the male, proboscis 780 long by 384 wide and 763 to 863 µm long by 374 to 426 µm wide in the female. The proboscis has 15 or 16 usually straight rows of between 9 and 11 hooks. In the male, the rooted hooks measure 29 to 30 µm, 41 to 47 µm, 55 to 57 µm, 55 to 56 µm from the anterior and the spings spiniform hooks measure 67 to 75 µm, 75 to 77 µm, 65 to 75 µm, 60 to 62 µm, 57 to 60 µm, 35 to 40 µm from the anterior. Proboscis receptacle 1.52 mm long by 0.52 mm wide in the male and 1.75 to 1.80 mm long by 0.47 to 0.55 mm wide in the female. The lemnisci are 18.75 mm long by 0.17 mm wide male and 13.75 to 16.25 mm long by 0.24 to 0.27 mm wide female.<ref name="Amin2014"/> |
|||
The male reproductive system is located in the posterior part of trunk. The testes are elongate with the anterior testis measuring between 3.50 and 6.87 mm long by 0.75 and 1.25 mm wide and the posterior testis measuring between 4.05 and 5.55 mm long by 0.95 and 1.32 mm wide. Cement glands are located posterior of the testes in cluster of 8 and measure between 0.87 and 2.50 mm long by 0.50 and 1.07 mm wide with the anterior glands being the largest. [[Saefftigen's pouch]] is 1.62 to 1.75 mm long by 0.50 to 0.57 mm wide. In the juvenile, the reproductive system is underdeveloped, with ovoid testes and occasionally lacking cement glands. The female reproductive system measures 572 to 946 µm in total length but lacks vaginal muscles. The uterus is thick and double-walled measuring 343 to 624 µm long and well-developed ligaments hold the distal end of uterus to body wall. The uterine bell is between 208 and 343 µm long and contains many nuclei. The eggs are ovoid being 73 to 114 µm long by 31 to 62 µm wide and are notched at one pole.<ref name="Amin2014">{{cite journal|doi=10.1654/4664.1|title=Description of ''Moniliformis kalahariensis'' (Acanthocephala: Moniliformidae) from the South African Hedgehog, ''Atelerix frontalis'' (Erinaceidae) in South Africa|year=2014|last1=Amin|first1=Omar M.|last2=Heckmann|first2=Richard A.|last3=Halajian|first3=Ali|last4=El-Naggar|first4=Atif|last5=Tavakol|first5=Sareh|journal=Comparative Parasitology|volume=81|pages=33–43}}</ref> |
|||
*'''''Moniliformis moniliformis''''' <small>(Bremser, 1811)</small>{{#tag:ref|''M. moniliformis'' originally named ''Echinorhynchus moniliformis'' by Bremser in 1811 but was renamed by Travassos in 1915. It is [[Synonym (taxonomy)|synonymous]] with ''E. grassi''<small>Railliet, 1893</small>, ''E. canis'' <small>Porter, 1914</small>, ''E. belgicus'' <small>Railliet, 1919</small>, ''M. dubius'' <small>Meyer, 1932</small>, and ''M. travassosi'' <small>Meyer, 1932</small><ref name="ITIS"/><ref name="Amin2013"/><ref name="Martins2017"/>|group=lower-alpha}} |
|||
{{Main|Moniliformis moniliformis}} |
|||
{|class="wikitable floatright" style="text-align: center; font-size: 75%;" |
|||
! colspan="3" |''M. moniliformis'' |
|||
|- |
|||
! Measurements<ref name="Martins2017"/>||Female (mm)||Male (mm) |
|||
|- |
|||
|Length of [[proboscis]]|||| |
|||
|- |
|||
|Width of [[proboscis]]|||| |
|||
|- |
|||
|Length of [[proboscis]] receptacle |||| |
|||
|- |
|||
|Width of [[proboscis]] receptacle |||| |
|||
|- |
|||
|Length of trunk|||| |
|||
|- |
|||
|Width of trunk|||| |
|||
|- |
|||
|Length of [[lemniscus (anatomy)|lemnisci]]|| || |
|||
|- |
|||
|Size of anterior testis||colspan="2" | |
|||
|- |
|||
|Size of posterior testis||colspan="2" | |
|||
|- |
|||
|Size of region occupied by cement glands||colspan="2" | |
|||
|- |
|||
|Size of eggs||colspan="2" | |
|||
|- |
|||
|Length of uterine bell||colspan="2" | |
|||
|- |
|||
|} |
|||
''M. moniliformis'' is a globally distributed parasite where the adult worms are usually found in intestines of [[rodent]]s or [[carnivore]]s such as cats and dogs but can on rare occasions infest humans. The [[Host (biology)#Types of hosts|intermediate hosts]] are usually [[beetle]]s and [[cockroach]]es.<ref name="Berenji2007"/> |
|||
The male averages 77 mm long and 1.66 mm wide whereas the female is much larger at 140 mm long and 1.24 mm wide.<ref name="Martins2017"/> The [[scolex]] of this worm has a cylindrical proboscis and a multitude of curved hooks. The proboscis of both genders are 0.50 mm long and between 1.19 and 1.24 mm wide and the proboscis receptacle is between 0.89 and 1.07 mm long and 0.27 and 0.28 mm wide. The proboscis has 11 to 12 longitudinal rows of hooks with 9 to 14 hooks per row with the largest hooks range from 20 to 30 mm long in the male and 31 mm long in the female.<ref name="Martins2017"/> Because of horizontal markings on the worm, there is the appearance of segmentation. The lemnisci are between 4.00 and 7.50 mm by 0.15 and 0.17 mm.<ref name="Martins2017"/> Males have testes that are between 2.20 and 3.00 mm by 0.50 to 0.80 mm, copulatory bursas used to hold on to the female during copulation, and eight cement glands that occupy an area of between 0.6 and 1.26 mm by 0.40 to 0.60 mm.<ref name = "Kansas State"/><ref name="Martins2017"/> Females have floating ovaries within a ligament sac where fertilization of the eggs, which are oval in shape with a thick, clear outer coat measuring between 0.11 and 0.12 mm long by 0.04 and 0.06 mm wide, occurs.<ref name="Martins2017"/><ref name = "Kansas State">{{cite web |url=http://www.k-state.edu/parasitology/classes/625acanth48.html |title=TOPIC 48. Phylum: Acanthocephala (thorny-headed worms) |
|||
|date=March 14, 2005 |publisher=Kansas State University|access-date= March 23, 2020}}</ref><ref name="CDC">{{cite web |url=https://www.cdc.gov/dpdx/acanthocephaliasis/index.html |title=Acanthocephaliasis |date=April 11, 2019 |website=Parasites and Health |publisher=Center for Disease Control |access-date=March 23, 2020}}</ref> Another report states the eggs also have 4 membranes and are 75 μm long and have an elongation ratio of 2.42.<ref name="eggs"/> |
|||
In the life cycle of ''M. moniliformis'', the intermediate hosts ingest the eggs of the parasite. In the intermediate host, the acanthor, or the parasite in its first larval stage, morphs into the acanthella, the second larval stage. After 6 to 12 weeks in this stage, the acanthella becomes a cystacanth. The cystacanth, or infective acanthella, of ''M. moniliformis'' are cyst-shaped and encyst in the tissues of the intermediate hosts in contrast to most other acanthocephalans which have infective larvae that more closely resemble underdeveloped adult worms. The definitive hosts consume the cystacanths upon feeding on infested intermediate hosts. These cystacanths mature and mate in the small intestine in 8 to 12 weeks. After this time, the eggs are excreted with the feces, to be ingested yet again by another intermediate host and renew this cycle.<ref name = "CDC" /> In what is commonly known as "brain-jacking," the parasite induces a behavioural change in its intermediate host that increases the risk of predation for the host. It is thought that this behavioral change holds an evolutionary advantage for the parasite by increasing its chances of getting to its definitive host. When ''M. moniliformis'' infests its intermediate host, the cockroach species ''[[Periplaneta americana]]'', it changes the cockroach's escape response. In one study, it was concluded that cockroaches infected by ''M. moniliformis'' took longer to respond to wind stimuli simulating the approach of a potential predator and displayed fewer escape responses implying that the parasite infection renders its intermediate host more vulnerable to predation by hindering its ability to detect and escape from its predator. It is thought that serotonin plays a role in upending the communication between giant interneurons and the thoracic interneurons and in turn hampers the escape response of the cockroach.<ref name="Berenji2007"/> In a similar study, the effects of parasitism on three ''[[Periplaneta]]'' species found that ''[[Periplaneta australasiae]]'' uses substrates differently and moves around less when infected with ''M. moniliformis''.<ref name = "Moore 1994">{{cite journal | last1 = Moore | first1 = J. | last2 = Freehling | first2 = M. | last3 = Gotelli | first3 = N. J. | year = 1994 | title = Altered behavior in two species of blattid cockroaches infected with ''Moniliformis moniliformis'' (Acanthocephala) | journal = Journal of Parasitology | volume = 80 | issue = 2 | pages = 220–223 | jstor=3283750 | doi=10.2307/3283750 | pmid = 8158464}}</ref> Another study concludes an increased vulnerability of infected ''Periplaneta americana'' due to increased [[phototaxis]], more time spent moving (due to slower movement) and movement in response to light (uninfected cockroaches hesitated before moving).<ref name="Moore 1983">{{cite journal |last1=Moore |first1=Janice |title=Altered Behavior in Cockroaches (Periplaneta americana) Infected with an Archiacanthocephalan, Moniliformis moniliformis |journal=The Journal of Parasitology |date=December 1983 |volume=69 |issue=6 |pages=1174–1176 |doi=10.2307/3280893 |jstor=3280893}}</ref> |
|||
Cases of moniliformiasis, a form of acanthocephaliasis, caused by human infestation by ''M. moniliformis'' have been reported in the United States, Iran, Iraq, and Nigeria,<ref name="Berenji2007">{{cite journal |last1=Berenji |first1=F |last2=Fata |first2=A|last3=Hosseininejad|first3=Z |date=2007|title=A case of ''Moniliformis moniliformis'' (Acanthocephala) infection in Iran |journal=Korean J Parasitol |volume=45 |issue=2 |pages=145–148 |doi=10.3347/kjp.2007.45.2.145 |pmid=17570979 |pmc=2526305}}</ref> but human moniliformiasis is now rare as it requires consumption of raw infested beetles or cockroaches. Calandruccio provided the first description of the clinical manifestations of moniliformiasis and similar accounts are found in the few case studies since; many of the patients described were asymptomatic. When they showed symptoms, they normally experienced abdominal pain, diarrhea, dizziness, edema, and anorexia.<ref name = "Richardson 2003">{{cite book|last1=Richardson|first1=Dennis J.|last2=Krause|first2=Peter J.|title=North American Parasitic Zoonoses|url=https://books.google.com/books?id=FJnlwOtlqAwC|volume=6|year=2003|publisher=Springer Science & Business Media|location=Boston|isbn=978-1-4020-7212-3}}</ref> In some patients, giddiness has also been reported.<ref name="Berenji2007"/> In rodents, moniliformiasis is fatal and manifests itself through hemorrhaging and gastrointestinal disturbance. |
|||
*'''''Moniliformis monoechinus''''' <small>(Linstow, 1902)</small>{{#tag:ref|''M. monoechinus'' was originally named ''Echinorhynchus monechinus'' by Linstow in 1902 but was given its present name by Petrochenko in 1958.<ref name="Amin2013"/> It is often incorrectly spelled ''M. monechinus''.<ref name="AminHeckmann2016"/>|group=lower-alpha}} |
|||
''M. monoechinus'' was found infesting the [[Giant anteater]] (''Myrmecophaga tridactyla'') in South America. The proboscis is very long measuring 3.55 mm long by 0.71 mm wide with 12 rows of 40 hooks each. The largest hooks are 130 µm long and found anteriorly and are shorter, 30 µm long, on the posterior portion.<ref name="AminHeckmann2016"/> |
|||
*'''''Moniliformis necromysi''''' <small>Gomes, Costa, Gentile, Vilela and Maldonado, 2020</small><ref name="Gomes2020">{{Cite journal |doi = 10.1017/S0022149X20000188|title = Morphological and genetic description of ''Moniliformis necromysi'' sp. n. (Archiacanthocephala) from the wild rodent Necromys lasiurus (Cricetidae: Sigmondontinae) in Brazil|year = 2020|last1 = Gomes|first1 = A.P.N.|last2 = Costa|first2 = N.A.|last3 = Gentile|first3 = R.|last4 = Vilela|first4 = R.V.|last5 = Maldonado|first5 = A.|journal = Journal of Helminthology|volume = 94|pages = e138|pmid = 32188515|s2cid = 213186167}}</ref> |
|||
''M. necromysi'' has been found infesting the [[hairy-tailed bolo mouse]] (''Necromys lasiurus'') in the [[cerrado]] in the state of [[Minas Gerais]], [[Brazil]]. [[Phylogenetic]] analyses using the partial mitochondrial [[cytochrome c oxidase subunit I]] gene and the nuclear [[LSU rRNA|large subunit ribosomal RNA]] gene demonstrated that this worm forms a well-supported monophyletic group within the genus ''Moniliformis''. The species name ''necromysi'' derives from the genus name of the host species ''Necromys''.<ref name="Gomes2020"/> |
|||
*'''''Moniliformis saudi''''' <small>Amin, Heckmann, Mohammed, & Evans, 2016</small><ref name="AminHeckmann2016">{{cite journal|last1=Amin|first1=Omar M.|last2=Heckmann|first2=Richard A.|last3=Osama|first3=Mohammed|last4=Evans|first4=R. Paul|title=Morphological and molecular descriptions of ''Moniliformis saudi'' sp. n. (Acanthocephala: Moniliformidae) from the desert hedgehog, ''Paraechinus aethiopicus'' (Ehrenberg) in Saudi Arabia, with a key to species and notes on histopathology|journal=Folia Parasitologica|volume=63|year=2016|issn=0015-5683|doi=10.14411/fp.2016.014|pmid=27189420|doi-access=free}}</ref> |
|||
[[File:Moniliformis saudi.jpg|thumb|The proboscis of ''Moniliformis saudi''<ref name="AminHeckmann2016"/>]] |
|||
''M. saudi'' has been found parasitizing the [[desert hedgehog]] (''Paraechinus aethiopicus'') in Saudi Arabia. The species was named after the country [[Saudi Arabia]] in which it was discovered. ''M. saudi'' is morphologically identical to ''M. cryptosaudi'', apart from the metal content of the hooks and genetic analysis.<ref name="Amin2019"/> |
|||
The trunk is between 24.00 and 50.00 mm long by 0.67 and 1.15 mm wide in the male and 18.00 to 117.50 mm long by 0.60 to 1.82 mm wide in the much larger female.<ref name="Amin2019"/> The anterior trunk tapers in a fan-shaped pattern and has a collar of vertical plates.<ref name="AminHeckmann2016"/> The proboscis is between 315 and 520 µm long by 130 and 177 µm wide in the male and 354 to 468 µm long by 130 to 208 µm wide in the female. The proboscis is armed with 13 to 14 rows of hooks each containing 7 or 8 hooks with the largest being found anteriorly and measuring 25 to 31 µm long in the female and 25 to 27 µm long in the male. The ventral hooks longer than dorsal hooks. The proboscis receptacle is 718 to 1250 µm by 155 to 322 µm in the female and 603 to 880 µm by 190 to 300 µm in the male. The long lemnisci and measures 2.91 to 8.75 mm by 0.08 to 0.17 mm in the male and measures 3.30 to 12.50 mm by 0.11 to 0.22 mm in the female. The short lemnisci and measures 2.81 to 5.75 mm by 0.07 to 0.14 mm in the male and measures 3.02 to 11.25 mm by 0.10 to 0.17 mm in the female. There are has 7 to 10 giant nuclei in the lemnisci. The anterior testes are 1.25 to 3.50 mm by 0.27 to 0.82 mm in size and the posterior testes are 1.12 to 3.50 mm by 0.27 to 0.65 mm in size. In the male, the cement glands are 312 to 811 µm by 270 to 468 µm and [[Saefftigen's pouch]] measures 468 to 1400 µm by 177 to 450 µm. In the female, The eggs are oval, measuring 57 to 83 µm by 31 to 42 µm.<ref name="Amin2019"/> |
|||
*'''''Moniliformis siciliensis''''' <small>Meyer, 1932</small>{{#tag:ref|''M. siciliensis'' is [[Synonym (taxonomy)|synonymous]] with ''M. pseudosegmentatus'' <small>(Knüppfer, 1888)</small><ref name="Amin2013"/>|group=lower-alpha}} |
|||
{|class="wikitable floatright" style="text-align: center; font-size: 75%;" |
|||
! colspan="3" |''M. siciliensis'' |
|||
|- |
|||
! Measurements<ref name="Martins2017"/>||Female (mm)||Male (mm) |
|||
|- |
|||
|Length of [[proboscis]]|||| |
|||
|- |
|||
|Width of [[proboscis]]|||| |
|||
|- |
|||
|Length of [[proboscis]] receptacle |||| |
|||
|- |
|||
|Width of [[proboscis]] receptacle |||| |
|||
|- |
|||
|Length of trunk|||| |
|||
|- |
|||
|Width of trunk|||| |
|||
|- |
|||
|Length of [[lemniscus (anatomy)|lemnisci]]|| || |
|||
|- |
|||
|Size of anterior testis||colspan="2" | |
|||
|- |
|||
|Size of posterior testis||colspan="2" | |
|||
|- |
|||
|Size of region occupied by cement glands||colspan="2" | |
|||
|- |
|||
|Size of eggs||colspan="2" | |
|||
|- |
|||
|Length of uterine bell||colspan="2" | |
|||
|- |
|||
|} |
|||
''M. siciliensis'' has been found infesting the [[brown rat]] (''Rattus norvegicus''<ref name="ITIS"/> reported as ''Mus decumanus''), and the [[garden dormouse]] (''Eliomys quercinus'') in [[Sicily]], [[Italy]]. The male is between 40 and 45 mm long by 1.0 and 1.50 mm wide whereas the female is much larger between 70 and 80 mm long by 1.0 and 1.50 mm wide. The proboscis is between 0.42 and 0.45 mm long by 0.17 to 0.19 mm wide in the male and female. The proboscis has 14 longitudinal rows of hooks with 8 hooks per row. The lemnisci are 10.0 by 0.17 mm. The eggs are oval being 0.08 mm long by 0.04 mm wide.<ref name="Martins2017"/> |
|||
*'''''Moniliformis spiralis''''' <small>Subrahmanian, 1927</small> |
|||
{|class="wikitable floatright" style="text-align: center; font-size: 75%;" |
|||
! colspan="3" |''M. spiralis'' |
|||
|- |
|||
! Measurements<ref name="Martins2017"/>||Female (mm)||Male (mm) |
|||
|- |
|||
|Length of [[proboscis]]|||| |
|||
|- |
|||
|Width of [[proboscis]]|||| |
|||
|- |
|||
|Length of [[proboscis]] receptacle |||| |
|||
|- |
|||
|Width of [[proboscis]] receptacle |||| |
|||
|- |
|||
|Length of trunk|||| |
|||
|- |
|||
|Width of trunk|||| |
|||
|- |
|||
|Length of [[lemniscus (anatomy)|lemnisci]]|| || |
|||
|- |
|||
|Size of anterior testis||colspan="2" | |
|||
|- |
|||
|Size of posterior testis||colspan="2" | |
|||
|- |
|||
|Size of region occupied by cement glands||colspan="2" | |
|||
|- |
|||
|Size of eggs||colspan="2" | |
|||
|- |
|||
|Length of uterine bell||colspan="2" | |
|||
|- |
|||
|} |
|||
''M. spiralis'' been found infesting ''[[Nesokia bengalensis]]'' and the [[black rat]] (''Rattus rattus'') in [[Rangoon]], [[Burma]].<ref name="Martins2017"/> It has also been found in [[India]] infesting a species of [[Bandicota]] and [[Herpestes]],<ref name="Sen1972">{{cite journal |last1=Sen |first1=J. K. |last2=Chauhan |first2=B. S. |date=1972 |title=On some acanthocephalan parasites from Indian mammals, with descriptions of two new species. |journal=Journal of the Zoological Society of India |volume=24 |issue=1 |pages=39–45}}</ref> and in bats in [[Kyrgyzstan]].<ref name="Logacheva1974">{{cite journal |last1=Logacheva |first1=L. S. |date=1974|title=Helminths of Chiroptera in Kirgizia |language=ru| publisher=Izdatel'stvo "ILIM". Frunze USSR|journal=Fauna Gel'mintov Zhivotnykh I Rastenii Kirgizii. |pages=49–51}}</ref> The male is 16.94 mm long and 0.56 mm wide whereas the female is much larger at 40 mm long and 0.91 mm wide. The proboscis of the male is 0.43 mm long and 0.11 mm wide and in the female is between 0.35 and 0.47 mm long and 0.17 to 0.18 mm wide. The proboscis receptacle of the male is 0.64 to 0.85 mm long and 0.24 to 0.30 mm wide and in the female it is 0.98 mm long and 0.31 mm wide. The proboscis has 12 to 14 longitudinal rows of hooks with 12 to 14 hooks per row with the largest hooks being 40 mm long. The lemnisci are 0.81 by 0.25 mm. The anterior testes are 0.81 by 0.25 mm and the posterior testes are 0.85 by 0.28 mm. There are six to eight cement glands 0.53 by 0.21 mm in size. The eggs are oval being 0.06 mm long by 0.03 mm wide and the genital pore is terminal and 0.91 by 0.48 mm.<ref name="Martins2017"/> |
|||
*'''''Moniliformis tarsii''''' <small>Deveaux, Schmidt and Krishnasamy, 1988</small><ref name="Deveaux1988"/> |
|||
''M. tarsii'' was found infesting the small and large intestines of [[Horsfield's tarsier]] (''Tarsius bancanus'') in [[Poring]], [[Sabah]], [[Malaysia]]. The male is 15 to 19 mm long and 800 to 900 µm wide whereas the female is larger being 21 to 41 mm long and 0.73 to 1.57 mm wide with a trunk lacking pseudosegmentation. The proboscis in the male is 490 μm long and 240 µm wide and in the female is 490 to 560 µm long and 230 to 320 µm wide. The proboscis is armed with 11 or 12 regularly alternating longitudinal rows of 6 or 7 hooks with the intermediate hooks being considerably larger than the others. Anterior hooks measure 26 to 37 µm, the next two hooks measure 41 to 55 µm, all three of which have powerful roots, whereas the remaining hooks four through seven measure 24 to 28 µm and have weak roots. The lemnsici are flat with three giant nuclei each and reach about 25–30% of the body length and are not bound posteriorly to the inner body wall. In the male, the anterior testes are 1.66 to 1.88 mm long and 330 µm wide, and the posterior testes are 1.5 mm long and 430 µm wide. The males also have eight cement glands each with a single giant nucleus located posterior to the testes. In the female, the uterine bell is 1.03 mm from posterior and eggs are very small and oval, being 47 to 55 µm long and 16 to 21 µm wide. The species name ''tarsii'' derives from the genus name of the host ''Tarsius''.<ref name="Deveaux1988"/> |
|||
*'''''Moniliformis travassosi''''' <small>Meyer, 1932</small> |
|||
{|class="wikitable floatright" style="text-align: center; font-size: 75%;" |
|||
! colspan="3" |''M. travassosi'' |
|||
|- |
|||
! Measurements<ref name="Martins2017"/>||Female (mm)||Male (mm) |
|||
|- |
|||
|Length of [[proboscis]]|||| |
|||
|- |
|||
|Width of [[proboscis]]|||| |
|||
|- |
|||
|Length of [[proboscis]] receptacle |||| |
|||
|- |
|||
|Width of [[proboscis]] receptacle |||| |
|||
|- |
|||
|Length of trunk|||| |
|||
|- |
|||
|Width of trunk|||| |
|||
|- |
|||
|Length of [[lemniscus (anatomy)|lemnisci]]|| || |
|||
|- |
|||
|Size of anterior testis||colspan="2" | |
|||
|- |
|||
|Size of posterior testis||colspan="2" | |
|||
|- |
|||
|Size of region occupied by cement glands||colspan="2" | |
|||
|- |
|||
|Size of eggs||colspan="2" | |
|||
|- |
|||
|Length of uterine bell||colspan="2" | |
|||
|- |
|||
|} |
|||
''M. travassosi'' was found infesting the [[brown rat]] (''Rattus norvegicus'') in [[Brazil]]. The male measures between 60 and 80 mm long by 1.0 and 1.5 mm wide whereas the female is between 100 and 110 mm long and 1.5 mm wide. The proboscis has 14 longitudinal rows of hooks with 15 hooks per row with the largest hooks being 24 to 28 mm long. The lemnisci are 10.0 mm. In the male, the testes are 2.50 to 3.0 mm by 0.80 mm and there are eight cement glands. The eggs are oval being 0.12 to 0.12 mm long by 0.07 to 0.07 mm wide.<ref name="Martins2017"/> It is named after [[Lauro Travassos]], a Brazilian helminthologist and entomologist who named the genus ''Moniliformis'' to which this species belongs. |
|||
==Hosts== |
|||
[[File:Acanthocephala LifeCycle lg.jpg|thumb|250px|alt=Diagram of the life cycle of Acanthocephala|Life cycle of Acanthocephala.<ref>{{cite web |
|||
| url = https://www.cdc.gov/dpdx/acanthocephaliasis/index.html |
|||
| title = Acanthocephaliasis |
|||
| last = CDC’s Division of Parasitic Diseases and Malaria |
|||
| date = April 11, 2019 |
|||
| website = www.cdc.gov |
|||
| publisher = [[Center for Disease Control]] |
|||
| access-date = July 17, 2023 |
|||
| quote = |
|||
| archive-date = 8 June 2023 |
|||
| archive-url = https://web.archive.org/web/20230608133736/https://www.cdc.gov/dpdx/acanthocephaliasis/index.html |
|||
| url-status = live |
|||
}}</ref>]] |
|||
The life cycle of an acanthocephalan consists of three stages beginning when an infective acanthor (development of an egg) is released from the intestines of the [[Host (biology)#Types of hosts|definitive host]] and then ingested by an arthropod, the [[Host (biology)#Types of hosts|intermediate host]]. The intermediate hosts of are usually beetles or cockroaches. When the acanthor [[molt]]s, the second stage called the acanthella begins. This stage involves penetrating the wall of the [[mesenteron]] or the intestine of the intermediate host and growing. The final stage is the infective cystacanth which is the [[larva]]l or juvenile state of an Acanthocephalan, differing from the adult only in size and stage of sexual development. The cystacanths within the intermediate hosts are consumed by the definitive host, usually attaching to the walls of the intestines, and as adults they reproduce sexually in the intestines. The acanthor are passed in the [[feces]] of the definitive host and the cycle repeats. There are no known [[Host_(biology)#Types_of_hosts|paratenic hosts]] (hosts where parasites infest but do not undergo larval development or sexual reproduction) for ''Moniliformis''.<ref>{{cite book |
|||
| last = Schmidt |
|||
| first = G.D. |
|||
| editor-last1 = Crompton |
|||
| editor-first1 = D.W.T. |
|||
| editor-last2 = Nickol |
|||
| editor-first2 = B.B. |
|||
| author-link = |
|||
| date = 1985 |
|||
| title = Biology of the Acanthocephala |
|||
| chapter = Development and life cycles |
|||
| url = https://core.ac.uk/download/pdf/17218255.pdf |
|||
| location = Cambridge |
|||
| publisher = Cambridge Univ. Press |
|||
| pages = 273–305 |
|||
| isbn = |
|||
| access-date = 16 July 2023 |
|||
| archive-date = 22 July 2023 |
|||
| archive-url = https://web.archive.org/web/20230722191034/https://core.ac.uk/download/pdf/17218255.pdf |
|||
| url-status = live |
|||
}}</ref> |
|||
''Moniliformidae'' species are found in the intestines parasitizing mammals and occasionally birds.<ref name="Amin1987" /> Intermediate hosts are mostly cockroaches but also other insect groups.<ref name="AminHeckmann2016"/> Infestation can cause moniliformiasis, which is characterized as [[lesions]] in the intestines, [[Abdominal distension|intestinal distension]], [[perforated ulcer]]s, [[enteritis]], [[Crypt (anatomy)|crypt]] [[hypertrophy]], [[goblet cell]] [[hyperplasia]], and occlusions of the intestinal tract in the [[gray squirrel]] (''Sciurus carolinensis'').<ref name="Singleton1993">{{cite journal |last1=Singleton |first1=Jeurel |last2=Richardson |first2=Dennis J. |last3=Lockhart |first3=J. Mitchell |title=Severe Moniliformiasis (Acanthocephala: Moniliformidae) in a Gray Squirrel, Sciurus carolinensis, from Arkansas, USA |journal=Journal of Wildlife Diseases |date=January 1993 |volume=29 |issue=1 |pages=165–168 |doi=10.7589/0090-3558-29.1.165 |pmid=8445783 |s2cid=10220897}}</ref> There are multiple reports of human infection from Australia, Iran, Iraq, Nigeria, Zimbabwe, Saudi Arabia and United States, all from ''Moniliformis moniliformis''.<ref name="Mathison2021">{{cite journal |last1=Mathison |first1=BA |display-authors=etal |title=Human Acanthocephaliasis: a Thorn in the Side of Parasite Diagnostics |journal=J Clin Microbiol |date=2021 |volume=59 |issue=11 |page=e02691-20 |doi=10.1128/JCM.02691-20 |pmid=34076470 |url=https://doi.org/10.1128%2FJCM.02691-20|pmc=8525584 }}</ref> |
|||
<gallery mode="packed" widths="180" caption="Hosts for ''Moniliformidae'' species"> |
|||
File:Common spiny mouse.JPG|alt=A Cairo spiny mouse on dirt|The [[Cairo spiny mouse]] is a host of ''Moniliformis acomysi'' |
|||
File:Four-toes-jerboa.jpg|alt=A Four-toed jerboa on a kitchen table with people in the background|The [[four-toed jerboa]] is a host of ''Moniliformis aegyptiacus'' |
|||
File:Atelerix algirus.jpg|alt=A North African hedgehog curled up on hay|The [[North African hedgehog]] is a host of ''Moniliformis aegyptiacus'' |
|||
File:Lisztaffe - Cottontop Tamarin - Saguinus oedipus.jpg|alt=A cotton-top tamarin on a tree stump in the jungle|The [[cotton-top tamarins]] is a host of ''Moniliformis clarki'' |
|||
File:Wilhelma Kalong-Flughund Pteropus vampyrus 0513.jpg|alt=A large flying fox hanging upsidedown on a branch|The [[large flying fox]] is a host of ''Moniliformis convolutus'' |
|||
File:Hemiechinus auritus; Baikonur 09.jpg|alt=Long-eared hedgehog on a wood floor in front of a wood door|The [[long-eared hedgehog]] is a host of ''Moniliformis cryptosaudi'' |
|||
File:Haarigel (Echinosorex gymnura).jpg|alt=A stuffed moonrat on display|The [[moonrat]] is a host of ''Moniliformis echinosorexi'' |
|||
File:Myresluger2.jpg|alt=A giant anteater|The [[giant anteater]] is a host of ''Moniliformis monoechinus'' |
|||
File:Desert Hedgehog.JPG|alt=A Desert hedgehog under branches|The [[desert hedgehog]] is a host of ''Moniliformis saudi'' |
|||
File:Rattus norvegicus -Fairlands Valley Park, Stevenage, England-8.jpg|alt=A brown rat on the ground|The [[brown rat]] is a host of ''M. siciliensis'' and ''Moniliformis travassosi'' |
|||
File:Eliomys quercinus01.jpg|alt=A Garden dormouse on a stone|The [[garden dormouse]] is a host of ''M. siciliensis'' |
|||
File:CSIRO ScienceImage 10564 The black rat Rattus rattus.jpg|alt=A black rat on the ground|The [[black rat]] is a host of ''Moniliformis spiralis'' |
|||
File:Bornean Tarsier (Cephalopachus bancanus borneanus) (8067063777).jpg|alt=Close-up of the face of Horsfield's tarsier on a branch|The [[Horsfield's tarsier]] is a host of ''Moniliformis tarsii'' |
|||
</gallery> |
|||
Although unusual, human infection by ''[[Moniliformis moniliformis]]'' has been observed in the United States<ref name="Neafie1993">{{Cite journal |
|||
| pmid = 8457979 |
|||
| year = 1993 |
|||
| last1 = Neafie | first1 = R. C. |
|||
| last2 = Marty |
|||
| title = Unusual infections in humans |
|||
| volume = 6 |
|||
| issue = 1 |
|||
| pages = 34–56 |
|||
| journal = Clinical Microbiology Reviews |
|||
| pmc = 358265 |
|||
}}</ref> and Iran.<ref>{{Cite journal |
|||
| last1 = Salehabadi | first1 = A. . |
|||
| last2 = Mowlavi | first2 = G. . |
|||
| last3 = Sadjjadi | first3 = S. . |
|||
| title = Human infection with ''Moniliformis moniliformis'' (Bremser 1811) (Travassos 1915) in Iran: another case report after three decades |
|||
| journal = Vector borne and zoonotic diseases (Larchmont, N.Y.) |
|||
| volume = 8 |
|||
| issue = 1 |
|||
| pages = 101–103 |
|||
| year = 2008 |
|||
| pmid = 18237263 |
|||
| doi = 10.1089/vbz.2007.0150 |
|||
}}</ref> Infection is known as acanthocephaliasis.<ref name="Neafie1993" /> |
|||
Contains the following species:{{#tag:ref|A [[Binomial nomenclature|binomial authority]] in parentheses indicates that the species was originally described in a genus other than ''Moniliformis''.|group=lower-alpha}} |
|||
*''[[Moniliformis acomysi]]'' <small>Ward and Nelson, 1967</small> |
|||
*''[[Moniliformis aegyptiacus]]'' <small>Meyer, 1932</small> |
|||
*''[[Moniliformis cestodiformis]]'' <small>([[Otto Friedrich Bernhard von Linstow|Linstow]], 1904)</small> |
|||
*''[[Moniliformis clarki]]'' <small>(Ward, 1917)</small> |
|||
*''[[Moniliformis convolutus]]'' <small>Meyer, 1932</small> |
|||
*''[[Moniliformis erinacei]]'' <small>Southwell and Macfie, 1925</small> |
|||
*''[[Moniliformis gracilis]]'' <small>(Rudolphi, 1819)</small> |
|||
*''[[Moniliformis kalahariensis]]'' <small>Meyer, 1931</small> |
|||
*''[[Moniliformis monechinus]]'' <small>(Linstow, 1902)</small> |
|||
*''[[Moniliformis moniliformis]]'' <small>(Bremser, 1811)</small> |
|||
*''[[Moniliformis saudi]]'' <small>Amin, Heckmann, Mohammed, & Evans, 2016</small> <ref name="AminHeckmann2016">{{cite journal|last1=Amin|first1=Omar M.|last2=Heckmann|first2=Richard A.|last3=Osama|first3=Mohammed|last4=Evans|first4=R. Paul|title=Morphological and molecular descriptions of ''Moniliformis saudi'' sp. n. (Acanthocephala: Moniliformidae) from the desert hedgehog, ''Paraechinus aethiopicus'' (Ehrenberg) in Saudi Arabia, with a key to species and notes on histopathology|journal=Folia Parasitologica|volume=63|year=2016|issn=0015-5683|doi=10.14411/fp.2016.014}} {{open access}}</ref> |
|||
*''[[Moniliformis semoni]]'' <small>(Linstow, 1898)</small> |
|||
*''[[Moniliformis spiradentatis]]'' <small>Mcleod, 1933</small> |
|||
*''[[Moniliformis spiralis]]'' <small>Subrahmanian, 1927</small> |
|||
*''[[Moniliformis travassosi]]'' <small>Meyer, 1932</small> |
|||
==Notes== |
==Notes== |
||
{{notelist}} |
{{notelist}} |
||
==References== |
==References== |
||
{{Reflist}} |
{{Reflist}} |
||
| Line 55: | Line 423: | ||
{{Taxonbar|from=Q2782820}} |
{{Taxonbar|from=Q2782820}} |
||
[[Category: |
[[Category:Archiacanthocephala]] |
||
[[Category:Acanthocephala genera]] |
|||
Revision as of 22:39, 13 January 2024
| Moniliformis | |
|---|---|
| |
| Adult Moniliformis moniliformis | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Acanthocephala |
| Class: | Archiacanthocephala |
| Order: | Moniliformida |
| Family: | Moniliformidae |
| Genus: | Moniliformis Travassos, 1915 |
Moniliformis is a genus of parasitic worms in the Acanthocephala phylum.
Taxonomy
Genetic analysis has been conducted on four species: Moniliformis moniliformis, M. saudi, M. cryptosaudi and M. kalahariensis.[1][2] Based on these results, Moniliformidae has been determined to be monophyletic.[1][2]
| Archiacanthocephala |
| Phylogenetic reconstruction for select species in the class Archiacanthocephala[3][4] |
Description
Species of the genus Moniliformis are usually pseudosegmented and have a cylindrical proboscis with longitudinal rows of hooks that have posteriorly directed roots. Moniliformis are further characterized by the presence of a simple, double-walled proboscis receptacle with the outer wall having spirally aligned muscle fibers, brain at posterior end of receptacle, and dorsal and ventral lacunar canals.[5][6] The proboscis retractor muscles pierce both the posterior and ventral end or just posterior end of the receptacle.[7] The cerebral ganglion is in the mid to posterior region, and the lemnisci are long and flat and not bound to the body wall. These worms also lack protonephridia and males have eight cement glands, each with a giant nucleus, which are used to temporarily close the posterior end of the female after copulation.[5][6][8]
Species
| M. acomysi | ||
|---|---|---|
| Measurements[9] | Female (mm) | Male (mm) |
| Length of proboscis | 0.20–0.43 | 0.19–0.36 |
| Width of proboscis | 0.14–0.22 | 0.12–0.21 |
| Length of proboscis receptacle | 0.68–0.84 | 0.68–0.84 |
| Width of proboscis receptacle | 0.24–0.33 | 0.24–0.33 |
| Length of trunk | 22–89 | 12–76 |
| Width of trunk | 0.56–1.50 | 0.26–1.50 |
| Size of lemnisci | 2.73–4.42 × 0.13–0.23 | 2.73–4.42 × 0.13–0.23 |
| Size of testis | 2.31 × 0.52–0.65 | |
| Size of cement glands | 0.300 | |
| Size of eggs | 0.07–0.09 x 0.03–0.05 | |
| M. aegyptiacus | ||
|---|---|---|
| Measurements[9] | Female (mm) | |
| Length of proboscis | 0.35 | |
| Width of proboscis | 0.16–0.18 | |
| Length of proboscis receptacle | 0.75 | |
| Length of trunk | 115 | |
| Length of lemnisci | 5.25–6.0 | |
| Size of eggs | 0.09–0.1 x 0.04–0.05 | |
| M. amini | ||
|---|---|---|
| Measurements[9] | Female (mm) | Male (mm) |
| Length of proboscis | 0.28–0.30 | 0.27 |
| Width of proboscis | 0.10–0.11 | 0.09 |
| Length of proboscis receptacle | 0.54–0.64 | 0.40 |
| Width of proboscis receptacle | 0.18–0.25 | 0.17 |
| Length of trunk | 63.96–120.83 | 20.21 |
| Width of trunk | 0.90–1.22 | 0.82 |
| Length of lemnisci | 6.90–11.90 | 5.40 |
| Size of anterior testis | 1.80 × 0.60 | |
| Size of posterior testis | 1.80 × 0.50 | |
| Size of region occupied by cement glands | 0.40 × 0.17 | |
| Size of eggs | 0.06 x 0.02 | |
| Length of uterine bell | 1.17 | |
| Distance from the uterine bell to genital pore | 1.08 | |
The genus Moniliformis Travassos, 1915 contains eighteen species.[10][11][a] Description of the genus is the same as the family Moniliformidae with the exception of possessing spiral muscles in the outer wall of the proboscis receptacle[5] and a single distinct kind of proboscis hooks.[12]
- Moniliformis acomysi Ward and Nelson, 1967[12]
M. acomysi was found infesting the Cairo spiny mouse (Acomys cahirinus) in South Sinai, Egypt.[12] The proboscis has eleven to sixteen longitudinal rows of hooks with six to ten hooks per row with the largest hooks being apical and range from 21 to 29 mm long in the male and 23 to 31 mm long in the female. There are eight cement glands in the male and the eggs of the female are oval.[9] The species name acomysi derives from the host genus name Acomys.[12]
- Moniliformis aegyptiacus Meyer, 1932[13]
M. aegyptiacus infests Meriones sinaiticus and the four-toed jerboa (Allactaga tetradactyla reported as Scarturus tetradactylus) in Egypt and the North African hedgehog (Atelerix algirus reported as Erinaceus algiris) in Tunis. The proboscis has 96 hooks arranged in twelve longitudinal rows with eight hooks per row. The eggs are oval.[9] The species name aegyptiacus derives from locality of the host, Egypt.[13]
- Moniliformis amini Martins, del Rosario Robles, and Navone 2017[9]
M. amini was found in the small intestine parasitising the olive grass mouse (Abrothrix olivaceus) in the Santa Cruz Province, Argentina. The species name amini was named after Omar Amin, a parasitologist specializing in Acanthocephala. Sexual dimorphism is pronounced with female over four times longer than the male. The proboscis has 12 to 14 longitudinal rows of hooks with 10 to 12 hooks per row with the largest hooks in the female being apical ranging from 22.21 to 24.47 μm, the middle being 15.50 μm long and the posterior being the smallest at 12 μm long. The largest hooks in the male are 24 μm. There are eight cement glands in the male, the eggs are oval.[9]
M. cestodiformis has been found infesting hedgehogs of the genus Erinaceus in West Africa. The proboscis is between 400 and 500 µm long and 200 µm wide with 16 to 18 rows of 7 or 8 hooks each and no apical pores. The largest hooks are found anteriorly and are 30 to 32 µm long with no dorsoventral differences.[1] The eggs have 4 membranes and are 85 to 92 µm long and 49 to 51 µm wide giving them an elongation ratio of 1.73.[1][16]
- Moniliformis clarki (Ward, 1917)[c]
| M. Clarki | ||
|---|---|---|
| Measurements[9] | Female (mm) | Male (mm) |
| Length of proboscis | ? | 0.41–0.52 |
| Width of proboscis | ? | 0.13–0.14 |
| Length of proboscis receptacle | ? | 0.65–0.67 |
| Width of proboscis receptacle | ? | 0.32–0.33 |
| Length of trunk | 120–250 | 61–85 |
| Width of trunk | 2.10–2.80 | 1.60–2.00 |
| Length of lemnisci | ? | 3.46–4.79 × 0.13–0.18 |
| Size of anterior testis | 3.30–5.62 × 0.72–1.15 | |
| Size of posterior testis | 3.97–4.29 × 0.72–1.17 | |
| Size of eggs | 0.05–0.09 x 0.03–0.0 | |
M. clarki commonly infects squirrels, chipmunks, deer mice, and gophers in North America.[1] If left untreated, it can kill its host. Ten cotton-top tamarins (Saguinus oedipus) were infected at the St. Louis Zoo with at least one being killed by the parasite.[17] Eggs of what is thought to be M. clarki due to the heavy population of common rodent hosts were discovered while examining human coprolite specimens gathered from Danger Cave in Utah. It was theorized that the man had either eaten a raw insect or an under cooked rodent.[18] The female is twice as long than the male. The proboscis has 12 or 13 longitudinal rows of hooks with six or seven hooks per row with the largest hooks being 23 to 28 mm long.[9] The eggs have 4 membranes and are 75 μm long and have an elongation ratio of 2.42.[16]
- Moniliformis convolutus Meyer, 1932
M. convolutus has been found infesting the large flying fox (Pteropus vampyrus) in Brazil. The proboscis is 500 µm long by 200 µm wide with 12 rows of 11 or 12 hooks each. The posterior hooks are rootless and the proboscis receptacle is 700 µm long.[1]
- Moniliformis cryptosaudi Amin, Heckmann, Sharifdini, and Albayati, 2019[2]
M. cryptosaudi is a cryptic species with M. saudi being morphologically identical, apart from the metal content of the hooks, but genetically distinct. The hooks consist of a collagen material with very low levels of phosphorus and calcium which is in contrast to those of M. saudi which has high levels of these two metals. The trunk is between 20.50 ad 48.75 mm long by 0.72 to 1.05 mm wide in the male and 28.75 to 121.25 mm long by 0.65 to 2.25 mm wide in the much larger female.[2] The anterior trunk tapers in a fan-shaped pattern and has a collar of vertical plates.[1] The proboscis is between 343 and 416 µm long by 135 and 188 µm wide in the male and 300 to 416 µm long by 162 to 291 µm wide in the female. The proboscis is armed with 13 to 14 rows of hooks each containing 7 or 8 hooks with the largest being found anteriorly and measuring 25 to 35 µm long in the female and 16 to 25 µm long in the male. The ventral hooks longer than dorsal hooks. The proboscis receptacle is 624 to 1092 µm by 218 to 364 µm in the female and 551 to 936 µm by 200 to 291 µm in the male. The long lemnisci has 6 to 8 giant nuclei and measures 4.26 to 7.28 mm by 0.12 to 0.18 mm in the male and has 6 to 9 giant nuclei and measures 4.15 to 8.12 mm by 0.11 to 0.21 mm in the female. The short lemnisci has 3 or 4 giant nuclei and measures 3.74 to 5.72 mm by 0.09 to 0.15 mm in the male and has 5 or 6 giant nuclei and measures 2.18 to 7.25 mm by 0.11 to 0.21 mm in the female. The anterior testes are 1.65 to 3.67 mm by 0.40 to 0.62 mm in size and the posterior testes are 1.50 to 2.62 mm by 0.37 to 0.62 mm in size. In the male, the cement glands are 322 to 780 µm by 229 to 416 µm and Saefftigen's pouch measures 624 to 925 µm by 270 to 425 µm. In the female, The eggs are oval, measuring 50 to 104 µm by 25 to 36 µm.[2]
The species name cryptosaudi refers to this cryptic status with M. saudi. It has been found in the intestine infesting the long-eared Hedgehog (Hemiechinus auritus) near Baqubah, Iraq.[2]
- Moniliformis echinosorexi Deveaux, Schmidt and Krishnasamy, 1988[19]
M. echinosorexi has been found infesting the moonrat (Echinosorex gymnurus) in Malaysia but may also infect hedgehogs. The worms have 12 to 15 rows of between 11 and 13 hooks that change from 34 to 36 μm long. The proboscis receptacle is 1.2 to 2.0 mm long.[19] Males are between 95 and 98 mm long whereas females are longer ranging from 126 to 192 mm long. The proboscis is relatively large ranging from 690 to 1000 µm long with 12 to 15 rows of containing 11 and 13 hooks each. The posterior hooks do not have roots. The proboscis receptacle is large at 1.2 mm long in males and 2.0 mm long in females. The testes are relatively large being between 5.6 and 6.8 mm long. Lemnisci are similarly long at with three giant nuclei each.[1] The species name echinosorexi derives from the genus of the host, Echinosorex.[19]
- Moniliformis gracilis (Rudolphi, 1819)[d]
M. gracilis is a parasite of the European roller (Coracias garrullus) in Europe. The worms are very small being only 2.25 to 3.00 mm long. The proboscis has 10 rows each with 8 or 9 hooks with the largest hooks on the anterior portion. The ganglion at the base of the short receptacle is 360 µm long.[1]
- Moniliformis ibunami[20]
- Moniliformis kalahariensis Meyer, 1931
M. kalahariensis was found infesting the Southern African Hedgehog (Atelerix frontalis) in Mohlonong and at the University of Limpopo in the Limpopo Province, South Africa.[21] and Namaqua sandgrouse (Pterocles namaqua) in Botswana. The intermediate host is the German cockroach (Blattella germanica).[22]
Juvelines are unsegmented whereas adults are pseudosegmented. There are 2 apical sensory pores on the proboscis. Males trunk is between 44.25 and 75.00 mm long by 1.62 and 1.75 mm wide whereas the females are larger being 51.25 to 81.25 mm long by 2.00 to 2.25 mm wide. In the male, proboscis 780 long by 384 wide and 763 to 863 µm long by 374 to 426 µm wide in the female. The proboscis has 15 or 16 usually straight rows of between 9 and 11 hooks. In the male, the rooted hooks measure 29 to 30 µm, 41 to 47 µm, 55 to 57 µm, 55 to 56 µm from the anterior and the spings spiniform hooks measure 67 to 75 µm, 75 to 77 µm, 65 to 75 µm, 60 to 62 µm, 57 to 60 µm, 35 to 40 µm from the anterior. Proboscis receptacle 1.52 mm long by 0.52 mm wide in the male and 1.75 to 1.80 mm long by 0.47 to 0.55 mm wide in the female. The lemnisci are 18.75 mm long by 0.17 mm wide male and 13.75 to 16.25 mm long by 0.24 to 0.27 mm wide female.[22]
The male reproductive system is located in the posterior part of trunk. The testes are elongate with the anterior testis measuring between 3.50 and 6.87 mm long by 0.75 and 1.25 mm wide and the posterior testis measuring between 4.05 and 5.55 mm long by 0.95 and 1.32 mm wide. Cement glands are located posterior of the testes in cluster of 8 and measure between 0.87 and 2.50 mm long by 0.50 and 1.07 mm wide with the anterior glands being the largest. Saefftigen's pouch is 1.62 to 1.75 mm long by 0.50 to 0.57 mm wide. In the juvenile, the reproductive system is underdeveloped, with ovoid testes and occasionally lacking cement glands. The female reproductive system measures 572 to 946 µm in total length but lacks vaginal muscles. The uterus is thick and double-walled measuring 343 to 624 µm long and well-developed ligaments hold the distal end of uterus to body wall. The uterine bell is between 208 and 343 µm long and contains many nuclei. The eggs are ovoid being 73 to 114 µm long by 31 to 62 µm wide and are notched at one pole.[22]
- Moniliformis moniliformis (Bremser, 1811)[e]
| M. moniliformis | ||
|---|---|---|
| Measurements[9] | Female (mm) | Male (mm) |
| Length of proboscis | ||
| Width of proboscis | ||
| Length of proboscis receptacle | ||
| Width of proboscis receptacle | ||
| Length of trunk | ||
| Width of trunk | ||
| Length of lemnisci | ||
| Size of anterior testis | ||
| Size of posterior testis | ||
| Size of region occupied by cement glands | ||
| Size of eggs | ||
| Length of uterine bell | ||
M. moniliformis is a globally distributed parasite where the adult worms are usually found in intestines of rodents or carnivores such as cats and dogs but can on rare occasions infest humans. The intermediate hosts are usually beetles and cockroaches.[23]
The male averages 77 mm long and 1.66 mm wide whereas the female is much larger at 140 mm long and 1.24 mm wide.[9] The scolex of this worm has a cylindrical proboscis and a multitude of curved hooks. The proboscis of both genders are 0.50 mm long and between 1.19 and 1.24 mm wide and the proboscis receptacle is between 0.89 and 1.07 mm long and 0.27 and 0.28 mm wide. The proboscis has 11 to 12 longitudinal rows of hooks with 9 to 14 hooks per row with the largest hooks range from 20 to 30 mm long in the male and 31 mm long in the female.[9] Because of horizontal markings on the worm, there is the appearance of segmentation. The lemnisci are between 4.00 and 7.50 mm by 0.15 and 0.17 mm.[9] Males have testes that are between 2.20 and 3.00 mm by 0.50 to 0.80 mm, copulatory bursas used to hold on to the female during copulation, and eight cement glands that occupy an area of between 0.6 and 1.26 mm by 0.40 to 0.60 mm.[24][9] Females have floating ovaries within a ligament sac where fertilization of the eggs, which are oval in shape with a thick, clear outer coat measuring between 0.11 and 0.12 mm long by 0.04 and 0.06 mm wide, occurs.[9][24][25] Another report states the eggs also have 4 membranes and are 75 μm long and have an elongation ratio of 2.42.[16]
In the life cycle of M. moniliformis, the intermediate hosts ingest the eggs of the parasite. In the intermediate host, the acanthor, or the parasite in its first larval stage, morphs into the acanthella, the second larval stage. After 6 to 12 weeks in this stage, the acanthella becomes a cystacanth. The cystacanth, or infective acanthella, of M. moniliformis are cyst-shaped and encyst in the tissues of the intermediate hosts in contrast to most other acanthocephalans which have infective larvae that more closely resemble underdeveloped adult worms. The definitive hosts consume the cystacanths upon feeding on infested intermediate hosts. These cystacanths mature and mate in the small intestine in 8 to 12 weeks. After this time, the eggs are excreted with the feces, to be ingested yet again by another intermediate host and renew this cycle.[25] In what is commonly known as "brain-jacking," the parasite induces a behavioural change in its intermediate host that increases the risk of predation for the host. It is thought that this behavioral change holds an evolutionary advantage for the parasite by increasing its chances of getting to its definitive host. When M. moniliformis infests its intermediate host, the cockroach species Periplaneta americana, it changes the cockroach's escape response. In one study, it was concluded that cockroaches infected by M. moniliformis took longer to respond to wind stimuli simulating the approach of a potential predator and displayed fewer escape responses implying that the parasite infection renders its intermediate host more vulnerable to predation by hindering its ability to detect and escape from its predator. It is thought that serotonin plays a role in upending the communication between giant interneurons and the thoracic interneurons and in turn hampers the escape response of the cockroach.[23] In a similar study, the effects of parasitism on three Periplaneta species found that Periplaneta australasiae uses substrates differently and moves around less when infected with M. moniliformis.[26] Another study concludes an increased vulnerability of infected Periplaneta americana due to increased phototaxis, more time spent moving (due to slower movement) and movement in response to light (uninfected cockroaches hesitated before moving).[27]
Cases of moniliformiasis, a form of acanthocephaliasis, caused by human infestation by M. moniliformis have been reported in the United States, Iran, Iraq, and Nigeria,[23] but human moniliformiasis is now rare as it requires consumption of raw infested beetles or cockroaches. Calandruccio provided the first description of the clinical manifestations of moniliformiasis and similar accounts are found in the few case studies since; many of the patients described were asymptomatic. When they showed symptoms, they normally experienced abdominal pain, diarrhea, dizziness, edema, and anorexia.[28] In some patients, giddiness has also been reported.[23] In rodents, moniliformiasis is fatal and manifests itself through hemorrhaging and gastrointestinal disturbance.
- Moniliformis monoechinus (Linstow, 1902)[f]
M. monoechinus was found infesting the Giant anteater (Myrmecophaga tridactyla) in South America. The proboscis is very long measuring 3.55 mm long by 0.71 mm wide with 12 rows of 40 hooks each. The largest hooks are 130 µm long and found anteriorly and are shorter, 30 µm long, on the posterior portion.[1]
- Moniliformis necromysi Gomes, Costa, Gentile, Vilela and Maldonado, 2020[29]
M. necromysi has been found infesting the hairy-tailed bolo mouse (Necromys lasiurus) in the cerrado in the state of Minas Gerais, Brazil. Phylogenetic analyses using the partial mitochondrial cytochrome c oxidase subunit I gene and the nuclear large subunit ribosomal RNA gene demonstrated that this worm forms a well-supported monophyletic group within the genus Moniliformis. The species name necromysi derives from the genus name of the host species Necromys.[29]
- Moniliformis saudi Amin, Heckmann, Mohammed, & Evans, 2016[1]

M. saudi has been found parasitizing the desert hedgehog (Paraechinus aethiopicus) in Saudi Arabia. The species was named after the country Saudi Arabia in which it was discovered. M. saudi is morphologically identical to M. cryptosaudi, apart from the metal content of the hooks and genetic analysis.[2]
The trunk is between 24.00 and 50.00 mm long by 0.67 and 1.15 mm wide in the male and 18.00 to 117.50 mm long by 0.60 to 1.82 mm wide in the much larger female.[2] The anterior trunk tapers in a fan-shaped pattern and has a collar of vertical plates.[1] The proboscis is between 315 and 520 µm long by 130 and 177 µm wide in the male and 354 to 468 µm long by 130 to 208 µm wide in the female. The proboscis is armed with 13 to 14 rows of hooks each containing 7 or 8 hooks with the largest being found anteriorly and measuring 25 to 31 µm long in the female and 25 to 27 µm long in the male. The ventral hooks longer than dorsal hooks. The proboscis receptacle is 718 to 1250 µm by 155 to 322 µm in the female and 603 to 880 µm by 190 to 300 µm in the male. The long lemnisci and measures 2.91 to 8.75 mm by 0.08 to 0.17 mm in the male and measures 3.30 to 12.50 mm by 0.11 to 0.22 mm in the female. The short lemnisci and measures 2.81 to 5.75 mm by 0.07 to 0.14 mm in the male and measures 3.02 to 11.25 mm by 0.10 to 0.17 mm in the female. There are has 7 to 10 giant nuclei in the lemnisci. The anterior testes are 1.25 to 3.50 mm by 0.27 to 0.82 mm in size and the posterior testes are 1.12 to 3.50 mm by 0.27 to 0.65 mm in size. In the male, the cement glands are 312 to 811 µm by 270 to 468 µm and Saefftigen's pouch measures 468 to 1400 µm by 177 to 450 µm. In the female, The eggs are oval, measuring 57 to 83 µm by 31 to 42 µm.[2]
- Moniliformis siciliensis Meyer, 1932[g]
| M. siciliensis | ||
|---|---|---|
| Measurements[9] | Female (mm) | Male (mm) |
| Length of proboscis | ||
| Width of proboscis | ||
| Length of proboscis receptacle | ||
| Width of proboscis receptacle | ||
| Length of trunk | ||
| Width of trunk | ||
| Length of lemnisci | ||
| Size of anterior testis | ||
| Size of posterior testis | ||
| Size of region occupied by cement glands | ||
| Size of eggs | ||
| Length of uterine bell | ||
M. siciliensis has been found infesting the brown rat (Rattus norvegicus[15] reported as Mus decumanus), and the garden dormouse (Eliomys quercinus) in Sicily, Italy. The male is between 40 and 45 mm long by 1.0 and 1.50 mm wide whereas the female is much larger between 70 and 80 mm long by 1.0 and 1.50 mm wide. The proboscis is between 0.42 and 0.45 mm long by 0.17 to 0.19 mm wide in the male and female. The proboscis has 14 longitudinal rows of hooks with 8 hooks per row. The lemnisci are 10.0 by 0.17 mm. The eggs are oval being 0.08 mm long by 0.04 mm wide.[9]
- Moniliformis spiralis Subrahmanian, 1927
| M. spiralis | ||
|---|---|---|
| Measurements[9] | Female (mm) | Male (mm) |
| Length of proboscis | ||
| Width of proboscis | ||
| Length of proboscis receptacle | ||
| Width of proboscis receptacle | ||
| Length of trunk | ||
| Width of trunk | ||
| Length of lemnisci | ||
| Size of anterior testis | ||
| Size of posterior testis | ||
| Size of region occupied by cement glands | ||
| Size of eggs | ||
| Length of uterine bell | ||
M. spiralis been found infesting Nesokia bengalensis and the black rat (Rattus rattus) in Rangoon, Burma.[9] It has also been found in India infesting a species of Bandicota and Herpestes,[30] and in bats in Kyrgyzstan.[31] The male is 16.94 mm long and 0.56 mm wide whereas the female is much larger at 40 mm long and 0.91 mm wide. The proboscis of the male is 0.43 mm long and 0.11 mm wide and in the female is between 0.35 and 0.47 mm long and 0.17 to 0.18 mm wide. The proboscis receptacle of the male is 0.64 to 0.85 mm long and 0.24 to 0.30 mm wide and in the female it is 0.98 mm long and 0.31 mm wide. The proboscis has 12 to 14 longitudinal rows of hooks with 12 to 14 hooks per row with the largest hooks being 40 mm long. The lemnisci are 0.81 by 0.25 mm. The anterior testes are 0.81 by 0.25 mm and the posterior testes are 0.85 by 0.28 mm. There are six to eight cement glands 0.53 by 0.21 mm in size. The eggs are oval being 0.06 mm long by 0.03 mm wide and the genital pore is terminal and 0.91 by 0.48 mm.[9]
- Moniliformis tarsii Deveaux, Schmidt and Krishnasamy, 1988[19]
M. tarsii was found infesting the small and large intestines of Horsfield's tarsier (Tarsius bancanus) in Poring, Sabah, Malaysia. The male is 15 to 19 mm long and 800 to 900 µm wide whereas the female is larger being 21 to 41 mm long and 0.73 to 1.57 mm wide with a trunk lacking pseudosegmentation. The proboscis in the male is 490 μm long and 240 µm wide and in the female is 490 to 560 µm long and 230 to 320 µm wide. The proboscis is armed with 11 or 12 regularly alternating longitudinal rows of 6 or 7 hooks with the intermediate hooks being considerably larger than the others. Anterior hooks measure 26 to 37 µm, the next two hooks measure 41 to 55 µm, all three of which have powerful roots, whereas the remaining hooks four through seven measure 24 to 28 µm and have weak roots. The lemnsici are flat with three giant nuclei each and reach about 25–30% of the body length and are not bound posteriorly to the inner body wall. In the male, the anterior testes are 1.66 to 1.88 mm long and 330 µm wide, and the posterior testes are 1.5 mm long and 430 µm wide. The males also have eight cement glands each with a single giant nucleus located posterior to the testes. In the female, the uterine bell is 1.03 mm from posterior and eggs are very small and oval, being 47 to 55 µm long and 16 to 21 µm wide. The species name tarsii derives from the genus name of the host Tarsius.[19]
- Moniliformis travassosi Meyer, 1932
| M. travassosi | ||
|---|---|---|
| Measurements[9] | Female (mm) | Male (mm) |
| Length of proboscis | ||
| Width of proboscis | ||
| Length of proboscis receptacle | ||
| Width of proboscis receptacle | ||
| Length of trunk | ||
| Width of trunk | ||
| Length of lemnisci | ||
| Size of anterior testis | ||
| Size of posterior testis | ||
| Size of region occupied by cement glands | ||
| Size of eggs | ||
| Length of uterine bell | ||
M. travassosi was found infesting the brown rat (Rattus norvegicus) in Brazil. The male measures between 60 and 80 mm long by 1.0 and 1.5 mm wide whereas the female is between 100 and 110 mm long and 1.5 mm wide. The proboscis has 14 longitudinal rows of hooks with 15 hooks per row with the largest hooks being 24 to 28 mm long. The lemnisci are 10.0 mm. In the male, the testes are 2.50 to 3.0 mm by 0.80 mm and there are eight cement glands. The eggs are oval being 0.12 to 0.12 mm long by 0.07 to 0.07 mm wide.[9] It is named after Lauro Travassos, a Brazilian helminthologist and entomologist who named the genus Moniliformis to which this species belongs.
Hosts

The life cycle of an acanthocephalan consists of three stages beginning when an infective acanthor (development of an egg) is released from the intestines of the definitive host and then ingested by an arthropod, the intermediate host. The intermediate hosts of are usually beetles or cockroaches. When the acanthor molts, the second stage called the acanthella begins. This stage involves penetrating the wall of the mesenteron or the intestine of the intermediate host and growing. The final stage is the infective cystacanth which is the larval or juvenile state of an Acanthocephalan, differing from the adult only in size and stage of sexual development. The cystacanths within the intermediate hosts are consumed by the definitive host, usually attaching to the walls of the intestines, and as adults they reproduce sexually in the intestines. The acanthor are passed in the feces of the definitive host and the cycle repeats. There are no known paratenic hosts (hosts where parasites infest but do not undergo larval development or sexual reproduction) for Moniliformis.[33]
Moniliformidae species are found in the intestines parasitizing mammals and occasionally birds.[7] Intermediate hosts are mostly cockroaches but also other insect groups.[1] Infestation can cause moniliformiasis, which is characterized as lesions in the intestines, intestinal distension, perforated ulcers, enteritis, crypt hypertrophy, goblet cell hyperplasia, and occlusions of the intestinal tract in the gray squirrel (Sciurus carolinensis).[34] There are multiple reports of human infection from Australia, Iran, Iraq, Nigeria, Zimbabwe, Saudi Arabia and United States, all from Moniliformis moniliformis.[35]
- Hosts for Moniliformidae species
-
The Cairo spiny mouse is a host of Moniliformis acomysi
-
The four-toed jerboa is a host of Moniliformis aegyptiacus
-
The North African hedgehog is a host of Moniliformis aegyptiacus
-
The cotton-top tamarins is a host of Moniliformis clarki
-
The large flying fox is a host of Moniliformis convolutus
-
The long-eared hedgehog is a host of Moniliformis cryptosaudi
-
The moonrat is a host of Moniliformis echinosorexi
-
The giant anteater is a host of Moniliformis monoechinus
-
The desert hedgehog is a host of Moniliformis saudi
-
The brown rat is a host of M. siciliensis and Moniliformis travassosi
-
The garden dormouse is a host of M. siciliensis
-
The black rat is a host of Moniliformis spiralis
-
The Horsfield's tarsier is a host of Moniliformis tarsii
Notes
- ^ A binomial authority in parentheses indicates that the species was originally described in a genus other than the present genus.
- ^ M. cestodiformis originally named Echinorhynchus cestodiformis by von Linstow in 1904[14] but was renamed by Travassos in 1917. It is synonymous with Moniliformis erinacei Southwell and Macfie, 1925[15][1]
- ^ M. clarki was originally named Hormorhynchus clarki by Ward in 1917 but was renamed by Chandler in 1921. It is synonymous with M. spiradentatis Mcleod, 1933.[15][1]
- ^ M. gracilis was originally named Echinorhynchus gracilis by Rudolphi in 1819 but was renamed by Meyer in 1931.[15][10]
- ^ M. moniliformis originally named Echinorhynchus moniliformis by Bremser in 1811 but was renamed by Travassos in 1915. It is synonymous with E. grassiRailliet, 1893, E. canis Porter, 1914, E. belgicus Railliet, 1919, M. dubius Meyer, 1932, and M. travassosi Meyer, 1932[15][10][9]
- ^ M. monoechinus was originally named Echinorhynchus monechinus by Linstow in 1902 but was given its present name by Petrochenko in 1958.[10] It is often incorrectly spelled M. monechinus.[1]
- ^ M. siciliensis is synonymous with M. pseudosegmentatus (Knüppfer, 1888)[10]
References
- ^ a b c d e f g h i j k l m n o p q Amin, Omar M.; Heckmann, Richard A.; Osama, Mohammed; Evans, R. Paul (2016). "Morphological and molecular descriptions of Moniliformis saudi sp. n. (Acanthocephala: Moniliformidae) from the desert hedgehog, Paraechinus aethiopicus (Ehrenberg) in Saudi Arabia, with a key to species and notes on histopathology". Folia Parasitologica. 63. doi:10.14411/fp.2016.014. ISSN 0015-5683. PMID 27189420.
- ^ a b c d e f g h i Amin, Omar M.; Heckmann, Richard A.; Sharifdini, Meysam; Albayati, Nagham Yaseen (2019). "Moniliformis cryptosaudi n. sp. (Acanthocephala: Moniliformidae) from the Long-eared Hedgehog Hemiechinus auritus (Gmelin) (Erinaceidae) in Iraq; A Case of Incipient Cryptic Speciation Related to M. saudi in Saudi Arabia". Acta Parasitologica. 64 (1): 195–204. doi:10.2478/s11686-018-00021-9. PMID 30666546. S2CID 58572640.
- ^ Nascimento Gomes, Ana Paula; Cesário, Clarice Silva; Olifiers, Natalie; de Cassia Bianchi, Rita; Maldonado, Arnaldo; Vilela, Roberto do Val (December 2019). "New morphological and genetic data of Gigantorhynchus echinodiscus (Diesing, 1851) (Acanthocephala: Archiacanthocephala) in the giant anteater Myrmecophaga tridactyla Linnaeus, 1758 (Pilosa: Myrmecophagidae)". International Journal for Parasitology: Parasites and Wildlife. 10: 281–288. doi:10.1016/j.ijppaw.2019.09.008. PMC 6906829. PMID 31867208.
- ^ Amin, O.M.; Sharifdini, M.; Heckmann, R.A.; Zarean, M. (2020). "New perspectives on Nephridiacanthus major (Acanthocephala: Oligacanthorhynchidae) collected from hedgehogs in Iran". Journal of Helminthology. 94: e133. doi:10.1017/S0022149X20000073. PMID 32114988. S2CID 211725160.
- ^ a b c Schmidt, Gerald D.; Edmonds, Stanley J. (1989). "Australiformis semoni (Linstow, 1898) n. Gen., n. Comb. (Acanthocephala: Moniliformidae) from Marsupials of Australia and New Guinea". The Journal of Parasitology. 75 (2): 215–7. doi:10.2307/3282769. JSTOR 3282769. PMID 2926590.
- ^ a b Kükenthal, W (2014). Gastrotricha and Gnathifera. Göttingen, Germany: Walter de Gruyter. p. 322. ISBN 978-3110274271.
- ^ a b Amin, O. M. (1987). "Key to the families and subfamilies of Acanthocephala, with the erection of a new class (Polyacanthocephala) and a new order (Polyacanthorhynchida)". Journal of Parasitology. 73 (6): 1216–1219. doi:10.2307/3282307. JSTOR 3282307. PMID 3437357.
- ^ Bush, Albert O.; Fernández, Jacqueline C.; Esch, Gerald W.; Seed, J. Richard (2001). Parasitism : the diversity and ecology of animal parasites. Cambridge, UK New York, NY: Cambridge University Press. p. 203. ISBN 0-521-66278-8. OCLC 44131774.
- ^ a b c d e f g h i j k l m n o p q r s t u v w Guerreiro Martins, Natalia Beatriz; del Rosario Robles, María; Navone, Graciela Teresa (August 2017). "A new species of Moniliformis from a Sigmodontinae rodent in Patagonia (Argentina)". Parasitology Research. 116 (8): 2091–2099. doi:10.1007/s00436-017-5508-9. PMID 28585077. S2CID 33203157.
- ^ a b c d e Amin, Omar M. (September 19, 2013). "Classification of the Acanthocephala". Folia Parasitologica. 60 (4): 273–305. doi:10.14411/fp.2013.031. PMID 24261131.
- ^ "Moniliformis Travassos, 1915". Integrated Taxonomic Information System (ITIS). November 3, 2023. Retrieved November 3, 2023.
- ^ a b c d Ward, Helen L.; Nelson, Diane R. (1967). "Acanthocephala of the Genus Moniliformis from Rodents of Egypt with the Description of a New Species from the Egyptian Spiny Mouse (Acomys cahirinus)". The Journal of Parasitology. 53 (1): 150–156. doi:10.2307/3276638. JSTOR 3276638. PMID 6066757.
- ^ a b Meyer, A. (1932). "Acanthocephala". Dr. H.G. Bronn's Klassen und Ordnungen des TierReichs (in German). 4. Akad. Verlag, Leipzig: 1–332.
- ^ von Linstow, O. (1904). "Neue Helminthen aus Westafrika". Centralbl. Bakt. Parasitenk., U. Lnfektionskr., Abt. 1 (36): 380–383.
- ^ a b c d e "Moniliformida Schmidt, 1972". Integrated Taxonomic Information System (ITIS). November 23, 2019. Retrieved January 30, 2020.
- ^ a b c Pfenning, Alana (20 August 2017). Egg morphology, dispersal, and transmission in acanthocephalan parasites: integrating phylogenetic and ecological approaches (Thesis).
- ^ Weber, M.; Junge, R. (2000). "Identification and treatment of Moniliformis clarki (Acanthocephala) in cotton-topped tamarins (Saguinus oedipus)". Journal of Zoo and Wildlife Medicine. 31 (4): 503–7. doi:10.1638/1042-7260(2000)031[0503:IATOMC]2.0.CO;2. PMID 11428397. S2CID 44668380.
- ^ Moore, J. G.; Fry, G. F.; Englert Jr, E. (1969). "Thomy-headed worm infection in north american prehistoric man". Science. 163 (3873): 1324–5. Bibcode:1969Sci...163.1324M. doi:10.1126/science.163.3873.1324. PMID 17807812. S2CID 6120428.
- ^ a b c d e Deveaux, Timothy P.; Schmidt, Gerald D.; Krishnasamy, M. (1988). "Two New Species of Moniliformis (Acanthocephala: Moniliformidae) from Malaysia". The Journal of Parasitology. 74 (2): 322–5. doi:10.2307/3282462. JSTOR 3282462. PMID 3128654.
- ^ Lynggaard, Christina; García-Prieto, Luis; Guzmán-Cornejo, Carmen; García-Varela, Martín (1 August 2021). "Description of a new species of Moniliformis (Acanthocephala: Moniliformidae) from Peromyscus hylocetes (Rodentia: Cricetidae) in Mexico". Parasitology International. 83: 102315. doi:10.1016/j.parint.2021.102315. PMID 33677125. S2CID 232142438.
- ^ Halajian, Ali; Warner, Lesley R.; Tavakol, Sareh; Smit, Nico J.; Luus-Powell, Wilmien J. (2018). "Checklist of acanthocephalan parasites of South Africa". ZooKeys (789): 1–18. Bibcode:2018ZooK..789....1H. doi:10.3897/zookeys.789.27710. PMC 6193052. PMID 30344432.
- ^ a b c Amin, Omar M.; Heckmann, Richard A.; Halajian, Ali; El-Naggar, Atif; Tavakol, Sareh (2014). "Description of Moniliformis kalahariensis (Acanthocephala: Moniliformidae) from the South African Hedgehog, Atelerix frontalis (Erinaceidae) in South Africa". Comparative Parasitology. 81: 33–43. doi:10.1654/4664.1.
- ^ a b c d Berenji, F; Fata, A; Hosseininejad, Z (2007). "A case of Moniliformis moniliformis (Acanthocephala) infection in Iran". Korean J Parasitol. 45 (2): 145–148. doi:10.3347/kjp.2007.45.2.145. PMC 2526305. PMID 17570979.
- ^ a b "TOPIC 48. Phylum: Acanthocephala (thorny-headed worms)". Kansas State University. March 14, 2005. Retrieved March 23, 2020.
- ^ a b "Acanthocephaliasis". Parasites and Health. Center for Disease Control. April 11, 2019. Retrieved March 23, 2020.
- ^ Moore, J.; Freehling, M.; Gotelli, N. J. (1994). "Altered behavior in two species of blattid cockroaches infected with Moniliformis moniliformis (Acanthocephala)". Journal of Parasitology. 80 (2): 220–223. doi:10.2307/3283750. JSTOR 3283750. PMID 8158464.
- ^ Moore, Janice (December 1983). "Altered Behavior in Cockroaches (Periplaneta americana) Infected with an Archiacanthocephalan, Moniliformis moniliformis". The Journal of Parasitology. 69 (6): 1174–1176. doi:10.2307/3280893. JSTOR 3280893.
- ^ Richardson, Dennis J.; Krause, Peter J. (2003). North American Parasitic Zoonoses. Vol. 6. Boston: Springer Science & Business Media. ISBN 978-1-4020-7212-3.
- ^ a b Gomes, A.P.N.; Costa, N.A.; Gentile, R.; Vilela, R.V.; Maldonado, A. (2020). "Morphological and genetic description of Moniliformis necromysi sp. n. (Archiacanthocephala) from the wild rodent Necromys lasiurus (Cricetidae: Sigmondontinae) in Brazil". Journal of Helminthology. 94: e138. doi:10.1017/S0022149X20000188. PMID 32188515. S2CID 213186167.
- ^ Sen, J. K.; Chauhan, B. S. (1972). "On some acanthocephalan parasites from Indian mammals, with descriptions of two new species". Journal of the Zoological Society of India. 24 (1): 39–45.
- ^ Logacheva, L. S. (1974). "Helminths of Chiroptera in Kirgizia". Fauna Gel'mintov Zhivotnykh I Rastenii Kirgizii. (in Russian). Izdatel'stvo "ILIM". Frunze USSR: 49–51.
- ^ CDC’s Division of Parasitic Diseases and Malaria (April 11, 2019). "Acanthocephaliasis". www.cdc.gov. Center for Disease Control. Archived from the original on 8 June 2023. Retrieved July 17, 2023.
- ^ Schmidt, G.D. (1985). "Development and life cycles". In Crompton, D.W.T.; Nickol, B.B. (eds.). Biology of the Acanthocephala (PDF). Cambridge: Cambridge Univ. Press. pp. 273–305. Archived (PDF) from the original on 22 July 2023. Retrieved 16 July 2023.
- ^ Singleton, Jeurel; Richardson, Dennis J.; Lockhart, J. Mitchell (January 1993). "Severe Moniliformiasis (Acanthocephala: Moniliformidae) in a Gray Squirrel, Sciurus carolinensis, from Arkansas, USA". Journal of Wildlife Diseases. 29 (1): 165–168. doi:10.7589/0090-3558-29.1.165. PMID 8445783. S2CID 10220897.
- ^ Mathison, BA; et al. (2021). "Human Acanthocephaliasis: a Thorn in the Side of Parasite Diagnostics". J Clin Microbiol. 59 (11): e02691-20. doi:10.1128/JCM.02691-20. PMC 8525584. PMID 34076470.













