Juan Nepomuceno Guerra and Osteoporosis: Difference between pages
Itsmejudith (talk | contribs) Wikified as part of Wikification wikiproject! |
image is not illustrative - his demographics make him very unlikely to have osteoporosis - commons page does not indicate that his consent has been obtained |
||
| Line 1: | Line 1: | ||
{{Infobox_Disease | |
|||
{{cleanup|date=October 2008}} |
|||
Name = Osteoporosis | |
|||
{{unreferenced|date=October 2008}} |
|||
Image = | |
|||
Caption = | |
|||
DiseasesDB = 9385 | |
|||
ICD10 = {{ICD10|M|80||m|80}}-{{ICD10|M|82||m|80}}| |
|||
ICD9 = {{ICD9|733.0}} | |
|||
ICDO = | |
|||
OMIM = 166710 | |
|||
MedlinePlus = 000360 | |
|||
eMedicineSubj = med | |
|||
eMedicineTopic = 1693 | |
|||
eMedicine_mult = {{eMedicine2|ped|1683}} {{eMedicine2|pmr|94}} {{eMedicine2|pmr|95}} | |
|||
MeshID = D010024 | |
|||
}} |
|||
'''Osteoporosis''' is a [[disease]] of [[bone]] that leads to an increased risk of [[bone fracture|fracture]]. In osteoporosis the [[bone mineral density]] (BMD) is reduced, bone microarchitecture is disrupted, and the amount and variety of [[collagen|non-collagenous]] proteins in bone is altered. Osteoporosis is defined by the [[World Health Organization]] (WHO) in women as a bone mineral density 2.5 [[standard deviation]]s below peak bone mass (20-year-old healthy female average) as measured by [[Dual energy X-ray absorptiometry|DXA]]; the term "established osteoporosis" includes the presence of a [[fragility fracture]].<ref name=WHO1994>{{cite journal |author=WHO |title=Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group |journal=World Health Organization technical report series |volume=843 |issue= |pages=1–129 |year=1994 |pmid=7941614 |doi=}}</ref> Osteoporosis is most common in women after [[menopause]], when it is called '''postmenopausal osteoporosis''', but may also develop in men, and may occur in anyone in the presence of particular hormonal disorders and other [[Chronic (medicine)|chronic]] diseases or as a result of [[medications]], specifically [[glucocorticoid]]s, when the disease is called '''steroid-''' or '''glucocorticoid-induced osteoporosis''' (SIOP or GIOP). Given its influence on the risk of fragility fracture, osteoporosis may significantly affect [[life expectancy]] and [[quality of life]]. |
|||
Osteoporosis can be prevented with lifestyle advice and sometimes medication, and in people with osteoporosis treatment may involve lifestyle advice, [[Fall prevention|preventing falls]] and medication ([[calcium in biology|calcium]], [[vitamin D]], [[bisphosphonate]]s and several others). |
|||
'''Juan Nepomuceno Guerra''' (died [[July 21]], [[2001]]) was a smuggler who founded the [[Gulf Cartel]]. During the 1930s he began smuggling whisky across the [[Mexican]]-[[United States]] border through south [[Texas]]. Through shrewd political connections he fostered he was able to control all the contraband moving across the [[Rio Grande]] River. In the 1970s his nephew, Juan Garcia Abgrego, began utilizing those connections and expanded the cartel into the more lucrative cocaine business.<ref>http://www.jornada.unam.mx/2003/03/15/046n1soc.php?origen=soc-jus.html</ref> |
|||
==Signs and symptoms== |
|||
Osteoporosis itself has [[asymptomatic|no specific symptoms]]; its main consequence is the increased risk of bone fractures. Osteoporotic [[fractures]] are those that occur in situations where healthy people would not normally break a bone; they are therefore regarded as ''[[fragility fracture]]s''. Typical fragility fractures occur in the [[vertebral column]], [[rib]], [[hip fracture|hip]] and [[wrist]]. |
|||
===Fractures=== |
|||
The symptoms of a [[vertebra]]l collapse ("[[compression fracture]]") are sudden [[back pain]], often with [[Radiculopathy|radiculopathic pain]] (shooting pain due to [[nerve]] compression) and rarely with [[spinal cord compression]] or [[cauda equina syndrome]]. Multiple vertebral fractures lead to a stooped posture, loss of height, and chronic pain with resultant reduction in mobility.<ref>{{cite journal |author=Kim DH, Vaccaro AR |title=Osteoporotic compression fractures of the spine; current options and considerations for treatment |journal=The spine journal : official journal of the North American Spine Society |volume=6 |issue=5 |pages=479–87 |year=2006 |pmid=16934715 |doi=10.1016/j.spinee.2006.04.013}}</ref> |
|||
Fractures of the long bones acutely impair mobility and may require [[surgery]]. [[Hip fracture]], in particular, usually requires prompt surgery, as there are serious risks associated with a hip fracture, such as [[deep vein thrombosis]] and a [[pulmonary embolism]], and increased mortality. |
|||
===Falls risk=== |
|||
The increased risk of falling associated with aging leads to fractures of the wrist, spine and hip. The risk of falling, in turn, is increased by impaired eyesight due to any cause (e.g. [[glaucoma]], [[macular degeneration]]), [[balance disorder]], [[movement disorder]]s (e.g. [[Parkinson's disease]]), [[dementia]], and [[sarcopenia]] (age-related loss of [[skeletal muscle]]). [[Collapse (medical)|Collapse]] (transient loss of postural tone with or without loss of consciousness) leads to a significant risk of falls; causes of syncope are manifold but may include [[cardiac arrhythmia]]s (irregular heart beat), [[vasovagal syncope]], [[orthostatic hypotension]] (abnormal drop in blood pressure on standing up) and [[seizure]]s. Removal of obstacles and loose carpets in the living environment may substantially reduce falls. Those with previous falls, as well as those with a gait or balance disorder, are most at risk.<ref>{{cite journal |author=Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ |title=Will my patient fall? |journal=JAMA |volume=297 |issue=1 |pages=77–86 |year=2007 |pmid=17200478 |doi=10.1001/jama.297.1.77}}</ref> |
|||
==Risk factors== |
|||
Risk factors for osteoporotic fracture can be split between non-modifiable and (potentially) modifiable. In addition, there are specific diseases and disorders in which osteoporosis is a recognized complication. Medication use is theoretically modifiable, although in many cases the use of medication that increases osteoporosis risk is unavoidable. |
|||
===Nonmodifiable=== |
|||
The most important risk factors for osteoporosis are advanced age (in both men and women) and [[female]] sex; [[estrogen]] deficiency following [[menopause]] is correlated with a rapid reduction in BMD, while in men a decrease in [[testosterone]] levels has a comparable (but less pronounced) effect. While osteoporosis occurs in people from all ethnic groups, [[European ethnic groups|European]] or [[Asian people|Asian]] ancestry predisposes for osteoporosis.<ref>{{cite journal |author=Melton LJ |title=Epidemiology worldwide |journal=Endocrinol. Metab. Clin. North Am. |volume=32 |issue=1 |pages=1–13, v |year=2003 |pmid=12699289 |doi=}}</ref> Those with a [[family history (medicine)|family history]] of fracture or osteoporosis are at an increased risk; the [[heritability]] of the fracture as well as low bone mineral density are relatively high, ranging from 25 to 80 percent. There are at least 30 genes associated with the development of osteoporosis.<ref name=Raisz/> Those who have already had a fracture are at least twice as likely to have another fracture compared to someone of the same age and sex.<ref>{{cite journal |author=Ojo F, Al Snih S, Ray LA, Raji MA, Markides KS |title=History of fractures as predictor of subsequent hip and nonhip fractures among older Mexican Americans |journal=Journal of the National Medical Association |volume=99 |issue=4 |pages=412–8 |year=2007 |pmid=17444431 |doi=}}</ref> |
|||
===Potentially modifiable=== |
|||
*Excess [[Alcoholic beverage|alcohol]] - small amounts of alcohol do not increase osteoporosis risk and may even be beneficial, but chronic heavy drinking(Alcohol intake greater than 2 units/day),<ref name="BMJosteoporosis">{{cite journal |author=Poole KE, Compston JE |title=Osteoporosis and its management |journal=BMJ |volume=333 |issue=7581 |pages=1251–6 |year=2006 |month=December |pmid=17170416 |doi=10.1136/bmj.39050.597350.47 |url=}}</ref> especially at a younger age, increases risk significantly.<ref>{{cite journal | author=Berg KM, Kunins HV, Jackson JL ''et al'' | title= Association between alcohol consumption and both osteoporotic fracture and bone density | journal=Am J Med | year=2008 | volume=121 | issue=5 | pages=406–18 | doi= 10.1016/j.amjmed.2007.12.012}}</ref> |
|||
*[[Vitamin D deficiency]]<ref name=micronutrients>{{cite journal |author=Nieves JW |title=Osteoporosis: the role of micronutrients. |journal=Am J Clin Nutr |volume=81 |issue=5 |pages=1232S–1239S |year=2005 |pmid=15883457 |url=http://www.ajcn.org/cgi/content/full/81/5/1232S}}</ref> - low circulating Vitamin D is common among the elderly worldwide.<ref name="WHOcriteria"/> Mild vitamin D insufficiency is associated with increased [[Parathyroid hormone|Parathyroid Hormone (PTH)]] production. <ref name="WHOcriteria"/> PTH increases bone reabsorption, leading to bone loss. A positive association exists between serum [[1,25-dihydroxycholecalciferol]] levels and bone mineral density, while PTH is negatively associated with bone mineral density.<ref name="WHOcriteria"/> |
|||
*[[Tobacco smoking]] - tobacco smoking inhibits the activity of osteoblasts, and is an independent risk factor for osteoporosis.<ref name="BMJosteoporosis"/><ref>{{cite journal |author=Wong PK, Christie JJ, Wark JD |title=The effects of smoking on bone health |journal=Clin. Sci. |volume=113 |issue=5 |pages=233–41 |year=2007 |pmid=17663660 |doi=10.1042/CS20060173| url=http://www.clinsci.org/cs/113/0233/cs1130233.htm}}</ref> Smoking also results in increased breakdown of exogenous estrogen, lower body weight and earlier menopause, all of which contribute to lower bone mineral density.<ref name="WHOcriteria"/> |
|||
*High [[body mass index]] - being overweight protects against osteoporosis, either by increasing load or through the hormone [[leptin]].<ref>{{cite journal |author=Shapses SA, Riedt CS |title=Bone, body weight, and weight reduction: what are the concerns? |journal=J. Nutr. |volume=136 |issue=6 |pages=1453–6 |year=2006 |pmid=16702302 |url=http://jn.nutrition.org/cgi/content/full/136/6/1453}}</ref> |
|||
*[[Malnutrition]] - low dietary [[calcium]] intake, low dietary intake of vitamins K and C<ref name=micronutrients /> Also low protein intake is associated with lower peak bone mass during adolescence and lower bone mineral density in elderly populations.<ref name="WHOcriteria"/> |
|||
* [[Physical exercise|Physical inactivity]] - [[bone remodeling]] occurs in response to physical stress. [[Weight bearing]] exercise can increase peak bone mass achieved in adolescence.<ref name="WHOcriteria"/> In adults, physical activity helps maintain bone mass, and can increase it by 1 or 2%. {{Fact|date=May 2008}} Conversely, physical inactivity can lead to significant bone loss.<ref name="WHOcriteria"/> |
|||
*Excess physical activity - excessive exercise can lead to constant damages to the bones which can cause exhaustion of the structures as described above. There are numerous examples of marathon runners who developed severe osteoporosis later in life. In women, heavy exercise can lead to decreased estrogen levels, which predisposes to osteoporosis. Intensive training is often associated with low body mass index. {{Fact|date=May 2008}} |
|||
*[[Heavy metals]] - a strong association between [[cadmium]], lead and bone disease has been established. Low level exposure to cadmium is associated with an increased loss of bone mineral density readily in both genders, leading to pain and increased risk of fractures, especially in the elderly and in females. Higher cadmium exposure results in [[osteomalacia]] (softening of the bone).<ref>{{cite journal | author = Staessen J, Roels H, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, Fagard R | title = Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group. | journal = Lancet | volume = 353 | issue = 9159 | pages = 1140–4 | year = 1999 | month = Apr 3 | pmid = 10209978 | doi = 10.1016/S0140-6736(98)09356-8 <!--Retrieved from CrossRef by DOI bot-->}}</ref> |
|||
*Soft drinks - some studies indicate that [[soft drink]]s (many of which contain [[phosphoric acid]]) may increase risk of osteoporosis;<ref>{{cite journal |author=Tucker KL, Morita K, Qiao N, Hannan MT, Cupples LA, Kiel DP |title=Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study |journal=Am. J. Clin. Nutr. |volume=84 |issue=4 |pages=936–42 |year=2006 |pmid=17023723 |doi=}}</ref> Others suggest soft drinks may displace calcium-containing drinks from the diet rather than directly causing osteoporosis.<ref>{{cite journal |author= |title=Soft drinks in schools |journal=Pediatrics |volume=113 |issue=1 Pt 1 |pages=152–4 |year=2004 |pmid=14702469 |doi=}}</ref> |
|||
===Diseases and disorders=== |
|||
Many diseases and disorders have been associated with osteoporosis.<ref name=ICSI>{{cite web |url=http://www.icsi.org/osteoporosis/diagnosis_and_treatment_of_osteoporosis__3.html |title=ICSI Health Care Guideline: Diagnosis and Treatment of Osteoporosis, 5th edition |accessdate=2008-04-08 |author=Simonelli, C ''et al'' |month=July|year=2006 |format=PDF |publisher=Institute for Clinical Systems Improvement}}</ref> For some, the underlying mechanism influencing the bone metabolism is straight-forward, whereas for others the causes are multiple or unknown. |
|||
*In general, [[immobilization]] causes bone loss (following the 'use it or lose it' rule). For example, localized osteoporosis can occur after prolonged immobilization of a fractured limb in a cast. This is also more common in active patients with a high bone turn-over (for example, athletes). Other examples include bone loss during [[space flight]] or in people who are bedridden or wheelchair-bound for various reasons. |
|||
*[[Hypogonadism|Hypogonadal]] states can cause secondary osteoporosis. These include [[Turner syndrome]], [[Klinefelter syndrome]], [[Kallmann syndrome]], [[anorexia nervosa]], [[andropause]]<ref name='medscapeosteoporosis'/>, [[hypothalamus|hypothalamic]] [[amenorrhea]] or [[hyperprolactinemia]]<ref name='medscapeosteoporosis'/>. In females, the effect of hypogonadism is mediated by [[estrogen]] deficiency. It can appear as early [[menopause]] (<45 years) or from prolonged premenopausal amenorrhea (>1 year). A bilateral [[oophorectomy]] (surgical removal of the ovaries) or a [[premature ovarian failure]] cause deficient estrogen production. In males, [[testosterone]] deficiency is the cause (for example, andropause or after surgical removal of the [[testes]]). |
|||
*Endocrine disorders that can induce bone loss include [[Cushing's syndrome]]<ref name="WHOcriteria"/>, [[hyperparathyroidism]]<ref name="WHOcriteria"/>, [[thyrotoxicosis]]<ref name="WHOcriteria"/>, [[hypothyroidism]], [[diabetes mellitus]] type 1 and 2,<ref name=OsteoporosisMen>{{cite journal|author=Ebeling PR|title=Clinical practice. Osteoporosis in men| journal=N Engl J Med| year=2008| volume=358| issue=14| pages=1474–82| pmid=18385499 |doi=10.1056/NEJMcp0707217}}</ref> [[acromegaly]] and [[adrenal insufficiency]]. In [[pregnancy]] and [[lactation]], there can be a reversible bone loss. <ref name=ICSI/> |
|||
*[[Malnutrition]], [[parenteral nutrition]]<ref name="WHOcriteria"/> and [[malabsorption]] can lead to osteoporosis. Nutritional and gastrointestinal disorders that can predispose to osteoporosis include [[coeliac disease]]<ref name="WHOcriteria"/>, [[Crohn's disease]], lactose intolerance, surgery<ref name='medscapeosteoporosis'/> (after [[gastrectomy]], [[intestinal bypass surgery]] or [[bowel resection]]) and severe [[liver disease]] (especially [[primary biliary cirrhosis]])<ref name='medscapeosteoporosis'/>. Patients with [[bulimia]] can also develop osteoporosis. Those with an otherwise adequate calcium intake can develop osteoporosis due to the inability to absorb calcium and/or vitamin D. Other micro-nutrients such as [[vitamin K]] or [[vitamin B12 deficiency]] may also contribute. |
|||
*Patients with rheumatologic disorders like [[rheumatoid arthritis]]<ref name='medscapeosteoporosis'/>, [[ankylosing spondylitis]]<ref name='medscapeosteoporosis'> {{cite web|url=http://www.medscape.com/viewarticle/427342 |title=Osteoporosis - Risk Factors, Screening, and Treatment |accessdate=2008-05-11 |last=Kohlmeier |first=Lynn Kohlmeier |year=1998 |publisher=Medscape Portals }}</ref>, [[systemic lupus erythematosus]] and polyarticular [[juvenile idiopathic arthritis]] are at increased risk of osteoporosis, either as part of their disease or because of other risk factors (notably corticosteroid therapy). Systemic diseases such as [[amyloidosis]] and [[sarcoidosis]] can also lead to osteoporosis. |
|||
*[[Renal insufficiency]] can lead to [[osteodystrophy]]. |
|||
*Hematologic disorders linked to osteoporosis are [[multiple myeloma]]<ref name='medscapeosteoporosis'/> and other [[monoclonal gammopathy|monoclonal gammopathies]],<ref name=OsteoporosisMen/> [[lymphoma]] and [[leukemia]], [[mastocytosis]]<ref name='medscapeosteoporosis'/>, [[hemophilia]], [[sickle-cell disease]] and [[thalassemia]]. |
|||
*Several inherited disorders have been linked to osteoporosis. These include [[osteogenesis imperfecta]]<ref name='medscapeosteoporosis'/>, [[Marfan syndrome]]<ref name='medscapeosteoporosis'/>, [[hemochromatosis]]<ref name="WHOcriteria"/>, [[hypophosphatasia]], [[glycogen storage disease]]s, [[homocystinuria]]<ref name='medscapeosteoporosis'/>, [[Ehlers-Danlos syndrome]]<ref name='medscapeosteoporosis'/>, [[porphyria]], [[Menkes disease|Menkes' syndrome]], [[epidermolysis bullosa]] and [[Gaucher's disease]]. |
|||
*People with [[scoliosis]] [[idiopathic|of unknown cause]] also have a higher risk of osteoporosis. Bone loss can be a feature of [[complex regional pain syndrome]]. It is also more frequent in people with [[Parkinson's disease]] and [[chronic obstructive pulmonary disease]]. |
|||
===Medication=== |
|||
Certain medications have been associated with an increase in osteoporosis risk; only steroids and anticonvulsants are classically associated, but evidence is emerging with regard to other drugs. |
|||
* Steroid-induced osteoporosis (SIOP) arises due to use of [[glucocorticoid]]s - analogous to Cushing's syndrome and involving mainly the axial skeleton. The synthetic glucocorticoid prescription drug [[prednisone]] is a main candidate after prolonged intake. Some professional guidelines recommend prophylaxis in patients who take the equivalent of more than 30 mg hydrocortisone (7.5 mg of prednisolone), especially when this is in excess of three months.<ref>{{cite book |author=Bone and Tooth Society of Great Britain, National Osteoporosis Society, Royal College of Physicians |title=Glucocorticoid-induced Osteoporosis |year=2003 |publisher=Royal College of Physicians of London |location=London, UK |isbn=1-860-16173-1 | url=http://www.rcplondon.ac.uk/pubs/contents/966c62dd-8011-4f65-a61d-dd0c7fe4fa4b.pdf}}</ref> Alternate day use may not prevent this complication.<ref name=GIOP>{{cite journal|author=Gourlay M, Franceschini N, Sheyn Y| title=Prevention and treatment strategies for glucocorticoid-induced osteoporotic fractures |journal=Clin Rheumatol| year=2007|volume=26|issue=2|pages=144–53|pmid=16670825 |doi=10.1007/s10067-006-0315-1}}</ref> |
|||
* [[Barbiturate]]s, [[phenytoin]] and some other enzyme-inducing [[antiepileptic]]s - these probably accelerate the metabolism of vitamin D. <ref>{{cite journal |author=Petty SJ, O'Brien TJ, Wark JD |title=Anti-epileptic medication and bone health |journal=Osteoporosis international |volume=18 |issue=2 |pages=129–42 |year=2007 |pmid=17091219 |doi=10.1007/s00198-006-0185-z}}</ref> |
|||
* [[L-Thyroxine]] over-replacement may contribute to osteoporosis, in a similar fashion as [[thyrotoxicosis]] does.<ref name="ICSI"/> This can be relevant in subclinical hypothyroidism. |
|||
* Several drugs induce [[hypogonadism]], for example [[aromatase inhibitors]] used in breast cancer, [[methotrexate]] and other anti-metabolite drugs, [[Depo-Provera|depot progesterone]] and [[gonadotropin-releasing hormone agonist]]s. |
|||
* [[Anticoagulant]]s - long-term use of heparin is associated with a decrease in bone density,<ref>{{cite journal |author=Ruiz-Irastorza G, Khamashta MA, Hughes GR |title=Heparin and osteoporosis during pregnancy: 2002 update |journal=Lupus |volume=11 |issue=10 |pages=680–2 |year=2002 |pmid=12413068| doi = 10.1191/0961203302lu262oa <!--Retrieved from CrossRef by DOI bot-->}}</ref> and [[warfarin]] (and related coumarins) have been linked with an increased risk in osteoporotic fracture in long-term use.<ref>{{cite journal |author=Gage BF, Birman-Deych E, Radford MJ, Nilasena DS, Binder EF |title=Risk of osteoporotic fracture in elderly patients taking warfarin: results from the National Registry of Atrial Fibrillation 2 |journal=Arch. Intern. Med. |volume=166 |issue=2 |pages=241–6 |year=2006 |pmid=16432096 |doi=10.1001/archinte.166.2.241|url=http://archinte.ama-assn.org/cgi/content/full/166/2/241}}</ref> |
|||
* [[Proton pump inhibitors]] - these drugs inhibit the production of [[gastric acid|stomach acid]]; it is thought that this interferes with calcium absorption.<ref>{{cite journal | author = Yang YX, Lewis JD, Epstein S, Metz DC | title=Long-term proton pump inhibitor therapy and risk of hip fracture | journal=JAMA | year=2006 | volume=296 | pages=2947–53 | pmid=17190895 | doi = 10.1001/jama.296.24.2947 <!--Retrieved from CrossRef by DOI bot-->}}</ref> Chronic [[phosphate]] binding may also occur with [[aluminium]]-containing [[antacids]].<ref name="ICSI"/> |
|||
* [[Thiazolidinedione]]s (used for diabetes) - [[rosiglitazone]] and possibly [[pioglitazone]], inhibitors of [[Peroxisome proliferator-activated receptor gamma|PPARγ]], have been linked with an increased risk of osteoporosis and fracture.<ref>{{cite journal |author=Murphy CE, Rodgers PT |title=Effects of thiazolidinediones on bone loss and fracture |journal=Ann Pharmacother |volume=41 |issue=12 |pages=2014–8 |year=2007 |pmid=17940125 |doi=10.1345/aph.1K286}}</ref> |
|||
*Chronic [[lithium]] therapy has been associated with osteoporosis.<ref name="ICSI"/> |
|||
==Diagnosis== |
|||
[[Image:Bone density scanner.jpg|right|thumb|A scanner used to measure bone density with [[dual energy X-ray absorptiometry]].]] |
|||
The diagnosis of osteoporosis is made on measuring the [[bone mineral density]] (BMD). The most popular method is dual energy X-ray absorptiometry (DXA or DEXA). In addition to the detection of abnormal BMD, the diagnosis of osteoporosis requires investigations into potentially modifiable underlying causes; this may be done with [[blood test]]s and [[X-ray]]s. Depending on the likelihood of an underlying problem, investigations for [[cancer]] with [[metastasis]] to the bone, [[multiple myeloma]], [[Cushing's disease]] and other above mentioned causes may be performed. |
|||
===Dual energy X-ray absorptiometry=== |
|||
[[Dual energy X-ray absorptiometry]] (DXA, formerly DEXA) is considered the [[gold standard (test)|gold standard]] for the diagnosis of osteoporosis. Osteoporosis is diagnosed when the [[bone mineral density]] is less than or equal to 2.5 standard deviations below that of a young adult reference population. This is translated as a [[Bone mineral density#T-score|T-score]]. The [[World Health Organization]] has established the following diagnostic guidelines:<ref name=WHO1994/><ref name="WHOcriteria">{{cite web | author=WHO Scientific Group on the Prevention and Management of Osteoporosis (2000 : Geneva, Switzerland) |url=http://whqlibdoc.who.int/trs/WHO_TRS_921.pdf |title=Prevention and management of osteoporosis : report of a WHO scientific group| year=2003 |accessdate=2007-05-31 |format=pdf |work=}}</ref> |
|||
* [[Bone mineral density#T-score|T-score]] -1.0 or greater is "normal" |
|||
* T-score between -1.0 and -2.5 is "low bone mass" (or "[[osteopenia]]") |
|||
* T-score -2.5 or below is osteoporosis |
|||
When there has also been an osteoporotic fracture (also termed "low trauma-fracture" or "fragility fracture"), defined as one that occurs as a result of a fall from a standing height, the term "severe or established" osteoporosis is used.<ref name=WHO1994/> |
|||
The International Society for Clinical Densitometry takes the position that a diagnosis of osteoporosis in men under 50 years of age should not be made on the basis of densitometric criteria alone. It also states that for pre-menopausal women, Z-scores (comparison with age group rather than peak bone mass) rather than T-scores should be used, and that the diagnosis of osteoporosis in such women also should not be made on the basis of densitometric criteria alone.<ref name="pmid14742881">{{cite journal |author=Leib ES, Lewiecki EM, Binkley N, Hamdy RC |title=Official positions of the International Society for Clinical Densitometry |journal=J Clin Densitom |volume=7 |issue=1 | pages = 1799 |year=2004 |pmid=14742881 | doi = 10.1385/JCD:7:1:1 <!--Retrieved from CrossRef by DOI bot-->}} quoted in: [http://www.guideline.gov/summary/summary.aspx?ss=15&doc_id=6567&nbr=4129 "Diagnosis of osteoporosis in men, premenopausal women, and children"]</ref> |
|||
===Screening=== |
|||
The [[U.S. Preventive Services Task Force]] (USPSTF) recommended in 2002 that all women 65 years of age or older should be [[screening (medicine)|screened]] with bone densitometry.<ref name="pmid12230355">{{cite journal |author=U.S. Preventive Services Task Force |title=Screening for osteoporosis in postmenopausal women: recommendations and rationale |journal=Ann. Intern. Med. |volume=137 |issue=6 |pages=526–8 |year=2002 |pmid=12230355 |doi=}}</ref> The Task Force recommends screening only those women ages 60 to 64 years of age who are at increased risk. The best risk factor for indicating increased risk is lower body weight (weight < 70 kg), with less evidence for smoking or family history. There was insufficient evidence to make recommendations about the optimal intervals for repeated screening and the appropriate age to stop screening. [[Clinical prediction rules]] are available to guide selection of women ages 60-64 for screening. The Osteoporosis Risk Assessment Instrument (ORAI) may be the most [[sensitivity (tests)|sensitive]] strategy<ref name="pmid17552058">{{cite journal |author=Martínez-Aguilà D, Gómez-Vaquero C, Rozadilla A, Romera M, Narváez J, Nolla JM |title=Decision rules for selecting women for bone mineral density testing: application in postmenopausal women referred to a bone densitometry unit |journal=J. Rheumatol. |volume=34 |issue=6 |pages=1307–12 |year=2007 |pmid=17552058 |doi=}}</ref> |
|||
Regarding the screening of men, a cost-analysis study suggests that screening may be "cost-effective for men with a self-reported prior fracture beginning at age 65 years and for men 80 years and older with no prior fracture".<ref name="pmid17684185">{{cite journal |author=Schousboe JT, Taylor BC, Fink HA, ''et al'' |title=Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men |journal=JAMA |volume=298 |issue=6 |pages=629–37 |year=2007 |pmid=17684185 |doi=10.1001/jama.298.6.629}}</ref> Also cost-effective is the screening of adult men from middle age on to detect any significant decrease in testosterone levels, say, below 300. |
|||
==Pathogenesis== |
|||
The underlying mechanism in all cases of osteoporosis is an imbalance between [[bone resorption]] and [[Bone#Formation|bone formation]]. In normal bone, there is constant [[matrix (biology)|matrix]] remodeling of bone; up to 10% of all bone mass may be undergoing remodeling at any point in time. The process takes place in bone multicellular units (BMUs) as first described by Frost in 1963.<ref>Frost HM, Thomas CC. Bone Remodeling Dynamics. Springfield, IL: 1963.</ref> Bone is resorbed by [[osteoclast]] cells (which derive from the [[bone marrow]]), after which new bone is deposited by [[osteoblast]] cells. <ref name=Raisz>{{cite journal | author = Raisz L | title = Pathogenesis of osteoporosis: concepts, conflicts, and prospects. | journal = J Clin Invest | volume = 115 | issue = 12 | pages = 3318–25 | year = 2005 | pmid = 16322775 | url=http://www.jci.org/cgi/content/full/115/12/3318 | doi=10.1172/JCI27071}}</ref> |
|||
The three main mechanisms by which osteoporosis develops are an inadequate ''peak bone mass'' (the skeleton develops insufficient mass and strength during growth), excessive bone resorption and inadequate formation of new bone during remodeling. An interplay of these three mechanisms underlies the development of fragile bone tissue.<ref name=Raisz/> Hormonal factors strongly determine the rate of bone resorption; lack of [[estrogen]] (e.g. as a result of menopause) increases bone resorption as well as decreasing the deposition of new bone that normally takes place in weight-bearing bones. The amount of estrogen needed to suppress this process is lower than that normally needed to stimulate the [[uterus]] and [[Mammary gland|breast gland]]. The α-form of the [[estrogen receptor]] appears to be the most important in regulating bone turnover.<ref name=Raisz/> In addition to estrogen, [[calcium metabolism]] plays a significant role in bone turnover, and deficiency of [[calcium in biology|calcium]] and [[vitamin D]] leads to impaired bone deposition; in addition, the [[parathyroid gland]]s react to low calcium levels by secreting [[parathyroid hormone]] (parathormone, PTH), which increases bone resorption to ensure sufficient calcium in the blood. The role of [[calcitonin]], a hormone generated by the [[thyroid]] that increases bone deposition, is less clear and probably not as significant as that of PTH.<ref name=Raisz/> |
|||
The activation of osteoclasts is regulated by various molecular signals, of which [[RANKL]] (receptor activator for [[NF-kB|nuclear factor κB]] ligand) is one of best studied. This molecule is produced by osteoblasts and other cells (e.g. [[lymphocyte]]s), and stimulates [[RANK]] (receptor activator of nuclear factor κB). [[Osteoprotegerin]] (OPG) binds RANKL before it has an opportunity to bind to RANK, and hence suppresses its ability to increase bone resorption. RANKL, RANK and OPG are closely related to [[tumor necrosis factor]] and its receptors. The role of the [[Wnt signaling pathway|''wnt'' signalling pathway]] is recognized but less well understood. Local production of [[eicosanoid]]s and [[interleukin]]s is thought to participate in the regulation of bone turnover, and excess or reduced production of these mediators may underlie the development of osteoporosis.<ref name=Raisz/> |
|||
[[Trabecular bone]] is the sponge-like bone in the ends of long bones and vertebrae. [[Cortical bone]] is the hard outer shell of bones and the middle of long bones. Because osteoblasts and osteoclasts inhabit the surface of bones, trabecular bone is more active, more subject to bone turnover, to remodeling. Not only is bone density decreased, but the microarchitecture of bone is disrupted. The weaker spicules of trabecular bone break ("microcracks"), and are replaced by weaker bone. Common osteoporotic fracture sites, the wrist, the hip and the spine, have a relatively high trabecular bone to cortical bone ratio. These areas rely on trabecular bone for strength, and therefore the intense remodeling causes these areas to degenerate most when the remodeling is imbalanced.{{Fact|date=September 2007}} |
|||
==Treatment== |
|||
There are several alternatives of medication to treat osteoporosis, depending on gender, though lifestyle changes are also very frequently an aspect of treatment. |
|||
===Medication=== |
|||
Bisphosphonates are the main pharmacological measures for treatment. However, newer drugs have appeared in the 1990s, such as teriparatide and strontium ranelate. |
|||
;Bisphosphonates |
|||
In confirmed osteoporosis, [[bisphosphonate]] drugs are the first-line treatment in women. The most often prescribed bisphosphonates are [[as of 2005|presently]] [[sodium alendronate]] (Fosamax) 10 mg a day or 70 mg once a week, [[risedronate]] (Actonel) 5 mg a day or 35 mg once a week and or [[ibandronate]] (Boniva) once a month. |
|||
A 2007 manufacturer-supported study suggested that in patients who had suffered a low-impact hip fracture, annual infusion of 5 mg [[Zoledronate|zoledronic acid]] reduced risk of any fracture by 35% (from 13.9 to 8.6%), vertebral fracture risk from 3.8% to 1.7% and non-vertebral fracture risk from 10.7% to 7.6%. This study also found a mortality benefit: after 1.9 years, 9.6% of the study group (as opposed to 13.3% of the control group) had died of any cause, indicating a mortality benefit of 28%.<ref>{{cite journal |author=Lyles KW, Colón-Emeric CS, Magaziner JS, ''et al'' |title=Zoledronic acid and clinical fractures and mortality after hip fracture |journal=N Engl J Med |volume= 357|issue= |pages=published online [[2007–09–17]] |year=2007 |pmid=17878149 |doi=10.1056/NEJMoa074941}}</ref> |
|||
Oral bisphosphonates are relatively poorly absorbed, and must therefore be taken on an empty stomach, with no food or drink to follow for the next 30 minutes. They are associated with [[esophagitis]] and are therefore sometimes poorly tolerated; weekly or monthly administration (depending on the preparation) decreases likelihood of esophagitis, and is now standard. Although intermittent dosing with the intravenous formulations such as zolendronate avoids oral tolerance problems, these agents are implicated at higher rates in a rare but unpleasant mouth disease called [[osteonecrosis of the jaw]].<ref>{{cite journal |author=Purcell, P. Boyd, I|title=Bisphosphonates and osteonecrosis of the jaw|journal=Medical Journal of Australia|volume=182 |issue=8 |pages=417–418 |year=2005 |pmid= |doi=}}</ref> For this reason, oral bisphosphonate therapy is probably to be preferred, and prescribing advice now recommends any remedial dental work to be carried out prior to commencing treatment.<ref>{{cite book |title=[[British National Formulary]] |chapter=6.6.2 Bisphosphonates |edition=54 |pages=p403 |month=September | year=2007 |publisher=[[British Medical Association]] and [[Royal Pharmaceutical Society of Great Britain]]}}</ref> |
|||
;Teriparatide |
|||
Recently, [[teriparatide]] (Forteo, [[recombinant]] [[parathyroid hormone]] residues 1–34) has been shown to be effective in osteoporosis. It acts like parathyroid hormone and stimulates osteoblasts, thus increasing their activity. It is used mostly for patients with established osteoporosis (who have already fractured), have particularly low BMD or several risk factors for fracture or cannot tolerate the oral bisphosphonates. It is given as a daily injection with the use of a pen-type injection device. Teriparatide is only licensed for treatment if bisphosphonates have failed or are contraindicated (however, this differs by country and is not required by the FDA in the USA. However, patients with previous radiation therapy, or Paget's disease, or young patients should avoid this medication). |
|||
;Strontium ranelate |
|||
Oral [[strontium ranelate]] is an alternative oral treatment, belonging to a class of drugs called "dual action bone agents" (DABAs) by its manufacturer. It has proven efficacy, especially in the prevention of vertebral fracture.<ref>{{cite journal |author=Meunier PJ, Roux C, Seeman E, ''et al'' |title=The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis |journal=N. Engl. J. Med. |volume=350 |issue=5 |pages=459–68 |year=2004 |pmid=14749454 |doi=10.1056/NEJMoa022436}}</ref> In laboratory experiments, strontium ranelate was noted to stimulate the proliferation of osteoblasts, as well as inhibiting the proliferation of osteoclasts. |
|||
Strontium ranelate is taken as a 2 g oral suspension daily, and is licenced for the treatment of osteoporosis to prevent vertebral and hip fracture. Strontium ranelate has side effect benefits over the bisphosphonates, as it does not cause any form of upper GI side effect, which is the most common cause for medication withdrawal in osteoporosis. In studies a small increase in the risk of [[venous thromboembolism]] was noted,<ref>{{cite journal |author=O'Donnell S, Cranney A, Wells GA, Adachi JD, Reginster JY |title=Strontium ranelate for preventing and treating postmenopausal osteoporosis |journal=Cochrane database of systematic reviews (Online) |volume= |issue=4 |pages=CD005326 |year=2006 |pmid=17054253 |doi=10.1002/14651858.CD005326.pub3}}</ref> the cause for which has not been determined. This suggests it may be less suitable in patients at risk for thrombosis for different reasons. The uptake of (heavier) strontium in place of calcium into bone matrix results in a substantial and disproportionate increase in bone mineral density as measured on DXA scanning<ref>{{cite journal |author=Reginster JY, Seeman E, De Vernejoul MC, ''et al'' |title=Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: treatment of peripheral osteoporosis (TROPOS) study. |journal=J Clin Endorinol Metab |volume=90 |issue= |pages=2816–22|year=2005 |pmid=15728210 |doi=}}</ref>, making further followup of bone density by this method harder to interpret for strontium treated patients. A correction algorithm has been devised.<ref>{{cite journal |author=Blake GM, Fogelman I |title=The correction of BMD measurements for bone strontium content |journal=J Clin Densitom |volume=10 |issue=3 |pages=259–65 |year=2007 |pmid=17543560 |doi=10.1016/j.jocd.2007.03.102}}</ref> |
|||
Although strontium ranelate is effective, it's not approved for use in the United States yet. However, strontium citrate is available in the U.S. from several well-known vitamin manufacturers. Most researchers believe that strontium is safe and effective no matter what form it's used. The ranelate form is simply a device invented by the Servier company of France so that they could patent their version of strontium.{{fact|date=April 2008}} |
|||
Strontium, no matter what the form, must be water-soluble and ionized in the stomach acid. Stontium is then protein-bound for transport from the intestinal tract into the blood stream. Unlike drugs like [[sodium alendronate]] (Fosamax), strontium doesn't inhibit bone recycling and, in fact, may produce stronger bones. Studies have shown that after five years alendronate may even cause bone loss, while strontium continues to build bone during lifetime use.{{fact|date=April 2008}} |
|||
Strontium must not be taken with food or calcium-containing preparations as calcium competes with strontium during uptake. However, it's essential that calcium, magnesium, and vitamin D in theraputic amounts must be taken daily, but not at the same time as strontium. Strontium should be taken on an empty stomach at night.{{fact|date=April 2008}} |
|||
;Hormone replacement |
|||
[[Estrogen replacement therapy]] remains a good treatment for prevention of osteoporosis but, at this time, is not recommended unless there are other indications for its use as well. There is uncertainty and controversy about whether estrogen should be recommended in women in the first decade after the menopause. |
|||
In hypogonadal men [[testosterone]] has been shown to give improvement in bone quantity and quality, but, as of 2008, there are no studies of the effects on fractures or in men with a normal testosterone level.<ref name="OsteoporosisMen"/> |
|||
;Selective estrogen receptor modulator (SERM) |
|||
SERMs are a class of medications that act on the estrogen receptors throughout the body in a selective manner. Normally, [[bone mineral density]] (BMD) is tightly regulated by a balanace between [[osteoblast]] and [[osteoclast]] activity in the trabecular bone. Estrogen has a major role in regulation of the bone formation-resorption equilibrium, as it stimulates osteoblast activity. Some SERMs such as [[raloxifene]] (Evista), act on the bone by slowing bone resorption by the osteoclasts.<ref>{{cite journal |author=Taranta A, Brama M, Teti A, ''et al'' |title=The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro |journal=Bone |volume=30 |issue=2 |pages=368–76 |year=2002 |month=February |pmid=11856644 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S8756328201006858}}</ref> SERMs have been proved as effective in clinical trials.<ref>{{cite journal |author=Meunier PJ, Vignot E, Garnero P, ''et al'' |title=Treatment of postmenopausal women with osteoporosis or low bone density with raloxifene. Raloxifene Study Group |journal=Osteoporos Int |volume=10 |issue=4 |pages=330–6 |year=1999 |pmid=10692984 |doi= |url=http://link.springer.de/link/service/journals/00198/bibs/9010004/90100330.htm}}</ref><ref>{{cite journal |author=Meunier PJ, Vignot E, Garnero P, ''et al'' |title=Treatment of postmenopausal women with osteoporosis or low bone density with raloxifene. Raloxifene Study Group |journal=Osteoporos Int |volume=10 |issue=4 |pages=330–6 |year=1999 |pmid=10692984 |doi= |url=http://link.springer.de/link/service/journals/00198/bibs/9010004/90100330.htm}}</ref> |
|||
===Nutrition=== |
|||
;Calcium |
|||
[[Calcium]] is required to support [[Ossification|bone growth]], [[bone healing]] and maintain bone strength and is one aspect of treatment for osteoporosis. Recommendations for calcium intake vary depending country and age; for individuals at higher risk of osteoporosis (after fifty years of age) the amount recommended by [[United States|US]] health agencies is 1,200 mg per day. Calcium supplements can be used to increase dietary intake, and absorption is optimized through taking in several small (500 mg or less) [[Dose (pharmacology)|doses]] throughout the day.<ref>{{cite web | url = http://www.niams.nih.gov/Health_Info/Bone/Bone_Health/Nutrition/default.asp | accessdate = 2008-01-28 | title = Nutrition and Bone Health | publisher = [[National Institute of Arthritis and Musculoskeletal and Skin Diseases|NIAMS]] | date = 2005-11-01 }}</ref> The role of calcium in preventing and treating osteoporosis is unclear - some populations with extremely low calcium intake also have extremely low rates of bone fracture, and others with high rates of calcium intake through [[milk]] and milk products have higher rates of bone fracture. Other factors, such as protein, salt and vitamin D intake, exercise and exposure to sunlight, can all influence bone mineralization, making calcium intake one factor among many in the development of osteoporosis.<ref name = Harvard>{{cite web | url = http://www.hsph.harvard.edu/nutritionsource/calcium.html | title = Calcium & Milk | publisher = Harvard School of Public Health | accessdate = 2008-01-28 | year = 2007 }}</ref> |
|||
A [[meta-analysis]] of [[randomized controlled trial]]s involving calcium and calcium plus vitamin D supported the use of high levels of calcium (1,200 mg or more) and vitamin D (800 IU or more), though outcomes varied depending on which measure was used to assess bone health (rates of fracture versus rates of bone loss).<ref name="pmid17720017">{{cite journal |author=Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A |title=Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis |journal=Lancet |volume=370 |issue=9588 |pages=657–66 |year=2007 |pmid=17720017 |doi=10.1016/S0140-6736(07)61342-7}}</ref> The meta-analysis, along with another study, also supported much better outcomes for patients with high [[Compliance (medicine)|compliance]] to the treatment protocol.<ref>{{cite journal |author=Prince RL, Devine A, Dhaliwal SS, Dick IM |title=Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women |journal=Arch. Intern. Med. |volume=166 |issue=8 |pages=869–75 |year=2006 |pmid=16636212 |doi=10.1001/archinte.166.8.869}}</ref> In contrast, despite earlier reports in improved [[high density lipoprotein]] (HDL, "good cholesterol") in calcium supplementation, a possible increase in the rate of [[myocardial infarction]] (heart attack) was found in a study in [[New Zealand]] in which 1471 women participated. If confirmed, this would indicate that calcium supplementation in women otherwise at low risk of fracture may cause more harm than good.<ref name="pmid18198394">{{cite journal |author=Bolland MJ, Barber PA, Doughty RN, ''et al'' |title=Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial |journal=BMJ |volume= 336|issue= | pages = 262|year=2008 |pmid=18198394 |doi=10.1136/bmj.39440.525752.BE}}</ref> |
|||
;Vitamin D |
|||
Some studies have shown that a high intake of [[vitamin D]] reduces fractures in the elderly,<ref name="pmid17720017"/><ref>{{cite journal |author=Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B |title=Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials |journal=JAMA |volume=293 |issue=18 |pages=2257–64 |year=2005 |pmid=15886381 |doi=10.1001/jama.293.18.2257}}</ref> though the [[Women's Health Initiative]] found that though calcium plus vitamin D did increase bone density, it did not affect hip fracture but did increase formation of [[kidney stone]]s.<ref>{{cite journal |author=Jackson RD, LaCroix AZ, Gass M, ''et al'' |title=Calcium plus vitamin D supplementation and the risk of fractures |journal=N. Engl. J. Med. |volume=354 |issue=7 |pages=669–83 |year=2006 |pmid=16481635 |doi=10.1056/NEJMoa055218}}</ref> |
|||
===Exercise=== |
|||
Multiple studies have shown that aerobics, weight bearing, and resistance exercises can all maintain or increase BMD in postmenopausal women.<ref>{{cite journal |author=Bonaiuti D, Shea B, Iovine R, ''et al'' |title=Exercise for preventing and treating osteoporosis in postmenopausal women |journal=Cochrane database of systematic reviews (Online) |volume= |issue=3 |pages=CD000333 |year=2002 |pmid=12137611| doi = 10.1002/14651858.CD000333 <!--Retrieved from CrossRef by DOI bot-->}}</ref> Many researchers have attempted to pinpoint which types of exercise are most effective at improving BMD and other metrics of bone quality, however results have varied. One year of regular jumping exercises appears to increase the BMD and [[moment of inertia]] of the proximal [[tibia]]<ref>{{cite journal |author=Cheng S, Sipilä S, Taaffe DR, Puolakka J, Suominen H |title=Change in bone mass distribution induced by hormone replacement therapy and high-impact physical exercise in post-menopausal women |journal=Bone |volume=31 |issue=1 |pages=126–35 |year=2002 |pmid=12110425| doi = 10.1016/S8756-3282(02)00794-9 <!--Retrieved from CrossRef by DOI bot-->}}</ref> in normal postmenopausal women. Treadmill walking, gymnastic training, stepping, jumping, endurance, and strength exercises all resulted in significant increases of L2-L4 BMD in osteopenic postmenopausal women.<ref>{{cite journal |author=Chien MY, Wu YT, Hsu AT, Yang RS, Lai JS |title=Efficacy of a 24-week aerobic exercise program for osteopenic postmenopausal women |journal=Calcif. Tissue Int. |volume=67 |issue=6 |pages=443–8 |year=2000 |pmid=11289692| doi = 10.1007/s002230001180 <!--Retrieved from CrossRef by DOI bot-->}}</ref><ref>{{cite journal |author=Iwamoto J, Takeda T, Ichimura S |title=Effect of exercise training and detraining on bone mineral density in postmenopausal women with osteoporosis |journal=Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association |volume=6 |issue=2 |pages=128–32 |year=2001 |pmid=11484097 |doi=10.1007/s0077610060128 |doi_brokendate=2008-06-25}}</ref><ref>{{cite journal |author=Kemmler W, Engelke K, Weineck J, Hensen J, Kalender WA |title=The Erlangen Fitness Osteoporosis Prevention Study: a controlled exercise trial in early postmenopausal women with low bone density-first-year results |journal=Archives of physical medicine and rehabilitation |volume=84 |issue=5 |pages=673–82 |year=2003 |pmid=12736880 |doi=}}</ref> Strength training elicited improvements specifically in [[distal]] [[radius]] and hip BMD.<ref>{{cite journal |author=Kerr D, Morton A, Dick I, Prince R |title=Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent |journal=J. Bone Miner. Res. |volume=11 |issue=2 |pages=218–25 |year=1996 |pmid=8822346 |doi=}}</ref> Exercise combined with other pharmacological treatments such as [[hormone replacement therapy]] (HRT) has been shown to increases BMD more than HRT alone.<ref name="Villareal2003">{{cite journal|author=Villareal DT, Binder EF, Yarasheski KE, et al|title=Effects of exercise training added to ongoing hormone replacement therapy on bone mineral density in frail elderly women|journal=J Am Geriatr Soc|year=2003|volume=51|issue=7|pages=985–90|pmid=12834519 | doi = 10.1046/j.1365-2389.2003.51312.x <!--Retrieved from CrossRef by DOI bot-->}}</ref> |
|||
Additional benefits for osteoporotic patients other than BMD increase include improvements in balance, gait, and a reduction in risk of falls.<ref name="Mayo2005">{{cite journal|author=Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR|title=Significant reduction in risk of falls and back pain in osteoporotic-kyphotic women through a Spinal Proprioceptive Extension Exercise Dynamic (SPEED) program|journal=Mayo Clin Proc|year=2005|volume=80|issue=7|pages=849–55|pmid=16007888}}</ref> |
|||
==Prognosis== |
|||
{| class="wikitable" align="right" |
|||
|+ Hip fractures per 1000 patient-years<ref name="pmid17846439">{{cite journal |author=Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD |title=Low bone mineral density and fracture burden in postmenopausal women |journal=CMAJ |volume=177 |issue=6 |pages=575–80 |year=2007 |pmid=17846439 |doi=10.1503/cmaj.070234}}</ref> |
|||
! WHO category !! Age 50-64 !! Age > 64 || Overall |
|||
|- |
|||
| Normal || 5.3 || 9.4 || 6.6 |
|||
|- |
|||
| [[Osteopenia]] || 11.4 || 19.6 || 15.7 |
|||
|- |
|||
| Osteoporosis || 22.4 || 46.6 || 40.6 |
|||
|} |
|||
Although osteoporosis patients have an increased mortality rate due to the complications of fracture, most patients die ''with'' the disease rather than ''of'' it. |
|||
Hip fractures can lead to decreased mobility and an additional risk of numerous complications (such as [[deep venous thrombosis]] and/or [[pulmonary embolism]], [[pneumonia]]). The 6-month mortality rate following hip fracture is approximately 13.5%, and a substantial proportion (almost 13%) of people who have suffered a hip fracture need total assistance to mobilize after a hip fracture.<ref>{{cite journal |author=Hannan EL, Magaziner J, Wang JJ, ''et al'' |title=Mortality and locomotion 6 months after hospitalization for hip fracture: risk factors and risk-adjusted hospital outcomes |journal=JAMA |volume=285 |issue=21 |pages=2736–42 |year=2001 |pmid=11386929| doi = 10.1001/jama.285.21.2736 <!--Retrieved from CrossRef by DOI bot-->}}</ref> |
|||
Vertebral fractures, while having a smaller impact on mortality, can lead to severe chronic pain of neurogenic origin, which can be hard to control, as well as deformity. Though rare, multiple vertebral fractures can lead to such severe hunch back ([[kyphosis]]) that the resulting pressure on internal organs can impair one's ability to breathe. |
|||
Apart from risk of death and other complications, osteoporotic fractures are associated with a reduced health-related [[quality of life]].<ref>{{cite journal |author=Brenneman SK, Barrett-Connor E, Sajjan S, Markson LE, Siris ES |title=Impact of recent fracture on health-related quality of life in postmenopausal women |journal=J. Bone Miner. Res. |volume=21 |issue=6 |pages=809–16 |year=2006 |pmid=16753011 |doi=10.1359/jbmr.060301}}</ref> |
|||
==Epidemiology== |
|||
[[Image:L1 2 vertebral fracture.jpg|right|thumb|Lateral thoraco-lumbar spine X-ray demonstrating multiple wedge fractures]] |
|||
It is estimated{{Fact|date=September 2007}} that 1 in 3 women and 1 in 12 men over the age of 50 worldwide have osteoporosis. It is responsible for millions of fractures annually, mostly involving the lumbar [[vertebrae]], [[hip]], and wrist. Fragility fractures of ribs are also common in men. |
|||
===Hip fractures=== |
|||
{{main|hip fractures}} |
|||
Hip fractures are responsible for the most serious consequences of osteoporosis. In the United States, more than 250,000 hip fractures annually are attributible to Osteoporosis.<ref name=RiggsEtAl2005>{{cite journal |
|||
| pmid = 8573428 |
|||
| title = The worldwide problem of osteoporosis: insights afforded by epidemiology. |
|||
| author = Riggs, B.L.; Melton, Lj 3.r.d. |
|||
| year = 2005 |
|||
| journal = Bone |
|||
}}</ref> It is estimated that a 50-year-old white woman has a 17.5% lifetime risk of fracture of the proximal [[femur]]. The incidence of hip fractures increases each decade from the sixth through the ninth for both women and men for all populations. The highest incidence is found among those men and women ages 80 or older.<ref name='Merkepid'/> |
|||
===Vertebral fractures=== |
|||
Between 35-50% of all women over 50 had at least one vertebral fracture. In the United States, 700,000 vertebral fractures occur annually, but only about a third are recognized. In a series of 9704 of women aged 68.8 on average studied for 15 years, 324 had already suffered a vertebral fracture at entry into the study; 18.2% developed a vertebral fracture, but that risk rose to 41.4% in women who had a previous vertebral fracture.<ref>{{cite journal | author = Cauley JA, Hochberg MC, Lui LY ''et al'' | year=2007 | title=Long-term Risk of Incident Vertebral Fractures |journal = JAMA |volume =298|pages=2761–2767|doi= 10.1001/jama.298.23.2761 | pmid=18165669}}</ref> |
|||
===Wrist=== |
|||
In the United States, 250,000 [[Distal radius fracture|wrist fractures]] annually are attributable to Osteoporosis.<ref name=RiggsEtAl2005/> Wrist fractures are the third most common type of osteoporotic fractures. The lifetime risk of sustaining a Colles' fracture is about 16% for white women. By the time women reach age 70, about 20% have had at least one wrist fracture.<ref name='Merkepid'> {{cite web|url=http://www.merckmedicus.com/pp/us/hcp/diseasemodules/osteoporosis/epidemiology.jsp |title=MerckMedicus Modules: Osteoporosis - Epidemiology |accessdate=2008-06-13 |publisher=Merck & Co., Inc }}</ref> |
|||
===Rib Fractures=== |
|||
Fragility fractures of the ribs are common in men as young as age thirty-five on. These are often overlooked as signs of osteoporosis as these men are often physically active and suffer the fracture in the course of physical activity. An example would be as a result of falling while water skiing or jet skiing. However, a quick test of the individual's testosterone level following the diagnosis of the fracture will readily reveal whether that individual might be at risk. |
|||
==Prevention== |
|||
Methods to prevent osteoporosis include changes of lifestyle. However, there are medications that can be used for prevention as well. As a different concept there are osteoporosis ortheses which help to prevent spine fractions and support the building up of muscles. [[Fall prevention]] can help prevent osteoporosis complications. |
|||
===Lifestyle=== |
|||
Lifestyle prevention of osteoporosis is in many aspects inversions from potentially modifiable risk factors. As [[tobacco smoking]] and unsafe [[alcoholic beverage|alcohol]] intake have been linked with osteoporosis, smoking cessation and moderation of alcohol intake are commonly recommended in the prevention of osteoporosis.{{fact|date=February 2008}} |
|||
;Exercise |
|||
Achieving a higher peak bone mass through exercise and proper nutrition during adolescence is important for the prevention of osteoporosis. Exercise and nutrition throughout the rest of the life delays bone degeneration. Jogging, walking, or stair climbing at 70-90% of maximum effort three times per week, along with 1,500 mg of calcium per day, increased bone density of the lumbar (lower) spine by 5% over 9 months. Individuals already diagnosed with osteopenia or osteoporosis should discuss their exercise program with their physician to avoid fractures.<ref name="pmid3259410">{{cite journal |author=Dalsky GP, Stocke KS, Ehsani AA, Slatopolsky E, Lee WC, Birge SJ |title=Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women |journal=Ann. Intern. Med. |volume=108 |issue=6 |pages=824–8 |year=1988 |pmid=3259410 |doi=}}</ref> |
|||
;Nutrition |
|||
A proper nutrition is a diet sufficient in [[calcium]] and [[vitamin D]]. Patients at risk for osteoporosis (e.g. [[steroid]] use) are generally treated with [[vitamin D]] and calcium supplements and often with bisphosphonates. In [[kidney|renal]] disease, more active forms of Vitamin D such as paracalcitol or (1,25-dihydroxycholecalciferol or [[calcitriol]] which is the main biologically active form of vitamin D) is used, as the kidney cannot adequately generate calcitriol from calcidiol (25-hydroxycholecalciferol) which is the storage form of vitamin D. |
|||
High [[Protein in nutrition|dietary protein]] intake increases calcium excretion in [[urine]] and has been linked to increased risk of fractures in research studies.<ref>{{cite journal |author=Feskanich D, Willett WC, Stampfer MJ, Colditz GA |title=Protein consumption and bone fractures in women |journal=Am. J. Epidemiol. |volume=143 |issue=5 |pages=472–9 |year=1996 |pmid=8610662 |doi=}}</ref> Other investigations have shown that protein is required for calcium absorption, but that excessive protein consumption inhibits this process. No interventional trials have been performed on dietary protein in the prevention and treatment of osteoporosis.<ref name="pmid12936953">{{cite journal |author=Kerstetter JE, O'Brien KO, Insogna KL |title=Dietary protein, calcium metabolism, and skeletal homeostasis revisited |journal=Am. J. Clin. Nutr. |volume=78 |issue=3 Suppl |pages=584S–592S |year=2003 |pmid=12936953 |doi=}}</ref> |
|||
===Medication=== |
|||
Just as for treatment, [[bisphosphonate]] can be used in cases of very high risk. Other medicines prescribed for prevention of osteoporosis include [[raloxifene]] (Evista), a [[selective estrogen receptor modulator]] (SERM). |
|||
[[Estrogen replacement therapy]] remains a good treatment for prevention of osteoporosis but, at this time, is not recommended unless there are other indications for its use as well. There is uncertainty and controversy about whether estrogen should be recommended in women in the first decade after the menopause. |
|||
In hypogonadal men [[testosterone]] has been shown to give improvement in bone quantity and quality, but, as of 2008, there are no studies of the effects on fractures or in men with a normal testosterone level.<ref name="OsteoporosisMen"/> |
|||
==History== |
|||
The link between age-related reductions in bone density and fracture risk goes back at least to [[Astley Cooper]], and the term "osteoporosis" and recognition of its pathological appearance is generally attributed to the French pathologist [[Jean Lobstein]].<ref>Lobstein JGCFM. ''Lehrbuch der pathologischen Anatomie.'' Stuttgart: Bd II, 1835.</ref> The American endocrinolgist [[Fuller Albright]] linked osteoporosis with the postmenopausal state.<ref>{{cite journal | author=Albright F, Bloomberg E, Smith PH |year=1940 |month= |title= Postmenopausal osteoporosis |journal=Trans. Assoc. Am. Physicians. |volume=55 |pages=298–305}}</ref> Bisphosponates, which revolutionized the treatment of osteoporosis, were discovered in the 1960s.<ref>{{cite journal |author=Patlak M |title=Bone builders: the discoveries behind preventing and treating osteoporosis |journal=FASEB J. |volume=15 |issue=10 |pages=1677E–E |year=2001 |pmid=11481214 |doi= 10.1096/fj.15.10.1677e}}</ref> |
|||
==See also== |
|||
*[[Back pain]] |
|||
*[[Hip protector]] |
|||
*[[Osteoporosis Orthesis]] |
|||
*[[Dental X-ray]] |
|||
*[[Osteopetrosis]] |
|||
*[[Osteoimmunology]] |
|||
*[[Ovariectomized rat]] model for osteoporosis research |
|||
==References== |
==References== |
||
{{Reflist}} |
{{Reflist|2}} |
||
==External links== |
|||
* {{dmoz|Health/Conditions_and_Diseases/Musculoskeletal_Disorders/Osteoporosis/}} |
|||
* [http://osteoed.org/tools.php Osteoporosis risk assessment tools] |
|||
* [http://www.who.int/nutrition/topics/5_population_nutrient/en/index25.html Diet, Nutrition and the prevention of osteoporosis] the [[World Health Organization]] and [[Food and Agriculture Organization]] (2003) |
|||
* [http://www.surgeongeneral.gov/library/bonehealth/ Bone Health and Osteoporosis: A Report of the Surgeon General] distributed by the U.S. [[Department of Health and Human Services]] |
|||
*[http://www.nof.org NOF.org] [[The National Osteoporosis Foundation]] |
|||
{{Osteochondropathy}} |
|||
[[Category:Aging-associated diseases]] |
|||
[[Category:Endocrinology]] |
|||
[[Category:Skeletal disorders]] |
|||
[[ar:هشاشة العظام]] |
|||
{{DEFAULTSORT:Nepomuceno Guerra, Juan}} |
|||
[[bg:Остеопороза]] |
|||
[[cs:Osteoporóza]] |
|||
[[da:Osteoporose]] |
|||
[[de:Osteoporose]] |
|||
[[el:Οστεοπόρωση]] |
|||
[[es:Osteoporosis]] |
|||
[[fr:Ostéoporose]] |
|||
[[ko:골다공증]] |
|||
[[hr:Osteoporoza]] |
|||
[[id:Osteoporosis]] |
|||
[[it:Osteoporosi]] |
|||
[[he:דלדול עצם]] |
|||
[[ka:ოსტეოპოროზი]] |
|||
[[la:Osteoporosis]] |
|||
[[lv:Osteoporoze]] |
|||
[[lt:Osteoporozė]] |
|||
[[nl:Osteoporose]] |
|||
[[ja:骨粗鬆症]] |
|||
[[no:Osteoporose]] |
|||
[[pl:Osteoporoza]] |
|||
[[pt:Osteoporose]] |
|||
[[ro:Osteoporoză]] |
|||
[[ru:Остеопороз]] |
|||
[[sl:Osteoporoza]] |
|||
[[su:Ostéoporosis]] |
|||
[[fi:Osteoporoosi]] |
|||
[[sv:Benskörhet]] |
|||
[[tr:Osteoporoz]] |
|||
[[uk:Остеопороз]] |
|||
[[zh:骨質疏鬆症]] |
|||
Revision as of 12:34, 10 October 2008
| Osteoporosis | |
|---|---|
| Specialty | Endocrinology |
Osteoporosis is a disease of bone that leads to an increased risk of fracture. In osteoporosis the bone mineral density (BMD) is reduced, bone microarchitecture is disrupted, and the amount and variety of non-collagenous proteins in bone is altered. Osteoporosis is defined by the World Health Organization (WHO) in women as a bone mineral density 2.5 standard deviations below peak bone mass (20-year-old healthy female average) as measured by DXA; the term "established osteoporosis" includes the presence of a fragility fracture.[1] Osteoporosis is most common in women after menopause, when it is called postmenopausal osteoporosis, but may also develop in men, and may occur in anyone in the presence of particular hormonal disorders and other chronic diseases or as a result of medications, specifically glucocorticoids, when the disease is called steroid- or glucocorticoid-induced osteoporosis (SIOP or GIOP). Given its influence on the risk of fragility fracture, osteoporosis may significantly affect life expectancy and quality of life.
Osteoporosis can be prevented with lifestyle advice and sometimes medication, and in people with osteoporosis treatment may involve lifestyle advice, preventing falls and medication (calcium, vitamin D, bisphosphonates and several others).
Signs and symptoms
Osteoporosis itself has no specific symptoms; its main consequence is the increased risk of bone fractures. Osteoporotic fractures are those that occur in situations where healthy people would not normally break a bone; they are therefore regarded as fragility fractures. Typical fragility fractures occur in the vertebral column, rib, hip and wrist.
Fractures
The symptoms of a vertebral collapse ("compression fracture") are sudden back pain, often with radiculopathic pain (shooting pain due to nerve compression) and rarely with spinal cord compression or cauda equina syndrome. Multiple vertebral fractures lead to a stooped posture, loss of height, and chronic pain with resultant reduction in mobility.[2]
Fractures of the long bones acutely impair mobility and may require surgery. Hip fracture, in particular, usually requires prompt surgery, as there are serious risks associated with a hip fracture, such as deep vein thrombosis and a pulmonary embolism, and increased mortality.
Falls risk
The increased risk of falling associated with aging leads to fractures of the wrist, spine and hip. The risk of falling, in turn, is increased by impaired eyesight due to any cause (e.g. glaucoma, macular degeneration), balance disorder, movement disorders (e.g. Parkinson's disease), dementia, and sarcopenia (age-related loss of skeletal muscle). Collapse (transient loss of postural tone with or without loss of consciousness) leads to a significant risk of falls; causes of syncope are manifold but may include cardiac arrhythmias (irregular heart beat), vasovagal syncope, orthostatic hypotension (abnormal drop in blood pressure on standing up) and seizures. Removal of obstacles and loose carpets in the living environment may substantially reduce falls. Those with previous falls, as well as those with a gait or balance disorder, are most at risk.[3]
Risk factors
Risk factors for osteoporotic fracture can be split between non-modifiable and (potentially) modifiable. In addition, there are specific diseases and disorders in which osteoporosis is a recognized complication. Medication use is theoretically modifiable, although in many cases the use of medication that increases osteoporosis risk is unavoidable.
Nonmodifiable
The most important risk factors for osteoporosis are advanced age (in both men and women) and female sex; estrogen deficiency following menopause is correlated with a rapid reduction in BMD, while in men a decrease in testosterone levels has a comparable (but less pronounced) effect. While osteoporosis occurs in people from all ethnic groups, European or Asian ancestry predisposes for osteoporosis.[4] Those with a family history of fracture or osteoporosis are at an increased risk; the heritability of the fracture as well as low bone mineral density are relatively high, ranging from 25 to 80 percent. There are at least 30 genes associated with the development of osteoporosis.[5] Those who have already had a fracture are at least twice as likely to have another fracture compared to someone of the same age and sex.[6]
Potentially modifiable
- Excess alcohol - small amounts of alcohol do not increase osteoporosis risk and may even be beneficial, but chronic heavy drinking(Alcohol intake greater than 2 units/day),[7] especially at a younger age, increases risk significantly.[8]
- Vitamin D deficiency[9] - low circulating Vitamin D is common among the elderly worldwide.[10] Mild vitamin D insufficiency is associated with increased Parathyroid Hormone (PTH) production. [10] PTH increases bone reabsorption, leading to bone loss. A positive association exists between serum 1,25-dihydroxycholecalciferol levels and bone mineral density, while PTH is negatively associated with bone mineral density.[10]
- Tobacco smoking - tobacco smoking inhibits the activity of osteoblasts, and is an independent risk factor for osteoporosis.[7][11] Smoking also results in increased breakdown of exogenous estrogen, lower body weight and earlier menopause, all of which contribute to lower bone mineral density.[10]
- High body mass index - being overweight protects against osteoporosis, either by increasing load or through the hormone leptin.[12]
- Malnutrition - low dietary calcium intake, low dietary intake of vitamins K and C[9] Also low protein intake is associated with lower peak bone mass during adolescence and lower bone mineral density in elderly populations.[10]
- Physical inactivity - bone remodeling occurs in response to physical stress. Weight bearing exercise can increase peak bone mass achieved in adolescence.[10] In adults, physical activity helps maintain bone mass, and can increase it by 1 or 2%. [citation needed] Conversely, physical inactivity can lead to significant bone loss.[10]
- Excess physical activity - excessive exercise can lead to constant damages to the bones which can cause exhaustion of the structures as described above. There are numerous examples of marathon runners who developed severe osteoporosis later in life. In women, heavy exercise can lead to decreased estrogen levels, which predisposes to osteoporosis. Intensive training is often associated with low body mass index. [citation needed]
- Heavy metals - a strong association between cadmium, lead and bone disease has been established. Low level exposure to cadmium is associated with an increased loss of bone mineral density readily in both genders, leading to pain and increased risk of fractures, especially in the elderly and in females. Higher cadmium exposure results in osteomalacia (softening of the bone).[13]
- Soft drinks - some studies indicate that soft drinks (many of which contain phosphoric acid) may increase risk of osteoporosis;[14] Others suggest soft drinks may displace calcium-containing drinks from the diet rather than directly causing osteoporosis.[15]
Diseases and disorders
Many diseases and disorders have been associated with osteoporosis.[16] For some, the underlying mechanism influencing the bone metabolism is straight-forward, whereas for others the causes are multiple or unknown.
- In general, immobilization causes bone loss (following the 'use it or lose it' rule). For example, localized osteoporosis can occur after prolonged immobilization of a fractured limb in a cast. This is also more common in active patients with a high bone turn-over (for example, athletes). Other examples include bone loss during space flight or in people who are bedridden or wheelchair-bound for various reasons.
- Hypogonadal states can cause secondary osteoporosis. These include Turner syndrome, Klinefelter syndrome, Kallmann syndrome, anorexia nervosa, andropause[17], hypothalamic amenorrhea or hyperprolactinemia[17]. In females, the effect of hypogonadism is mediated by estrogen deficiency. It can appear as early menopause (<45 years) or from prolonged premenopausal amenorrhea (>1 year). A bilateral oophorectomy (surgical removal of the ovaries) or a premature ovarian failure cause deficient estrogen production. In males, testosterone deficiency is the cause (for example, andropause or after surgical removal of the testes).
- Endocrine disorders that can induce bone loss include Cushing's syndrome[10], hyperparathyroidism[10], thyrotoxicosis[10], hypothyroidism, diabetes mellitus type 1 and 2,[18] acromegaly and adrenal insufficiency. In pregnancy and lactation, there can be a reversible bone loss. [16]
- Malnutrition, parenteral nutrition[10] and malabsorption can lead to osteoporosis. Nutritional and gastrointestinal disorders that can predispose to osteoporosis include coeliac disease[10], Crohn's disease, lactose intolerance, surgery[17] (after gastrectomy, intestinal bypass surgery or bowel resection) and severe liver disease (especially primary biliary cirrhosis)[17]. Patients with bulimia can also develop osteoporosis. Those with an otherwise adequate calcium intake can develop osteoporosis due to the inability to absorb calcium and/or vitamin D. Other micro-nutrients such as vitamin K or vitamin B12 deficiency may also contribute.
- Patients with rheumatologic disorders like rheumatoid arthritis[17], ankylosing spondylitis[17], systemic lupus erythematosus and polyarticular juvenile idiopathic arthritis are at increased risk of osteoporosis, either as part of their disease or because of other risk factors (notably corticosteroid therapy). Systemic diseases such as amyloidosis and sarcoidosis can also lead to osteoporosis.
- Renal insufficiency can lead to osteodystrophy.
- Hematologic disorders linked to osteoporosis are multiple myeloma[17] and other monoclonal gammopathies,[18] lymphoma and leukemia, mastocytosis[17], hemophilia, sickle-cell disease and thalassemia.
- Several inherited disorders have been linked to osteoporosis. These include osteogenesis imperfecta[17], Marfan syndrome[17], hemochromatosis[10], hypophosphatasia, glycogen storage diseases, homocystinuria[17], Ehlers-Danlos syndrome[17], porphyria, Menkes' syndrome, epidermolysis bullosa and Gaucher's disease.
- People with scoliosis of unknown cause also have a higher risk of osteoporosis. Bone loss can be a feature of complex regional pain syndrome. It is also more frequent in people with Parkinson's disease and chronic obstructive pulmonary disease.
Medication
Certain medications have been associated with an increase in osteoporosis risk; only steroids and anticonvulsants are classically associated, but evidence is emerging with regard to other drugs.
- Steroid-induced osteoporosis (SIOP) arises due to use of glucocorticoids - analogous to Cushing's syndrome and involving mainly the axial skeleton. The synthetic glucocorticoid prescription drug prednisone is a main candidate after prolonged intake. Some professional guidelines recommend prophylaxis in patients who take the equivalent of more than 30 mg hydrocortisone (7.5 mg of prednisolone), especially when this is in excess of three months.[19] Alternate day use may not prevent this complication.[20]
- Barbiturates, phenytoin and some other enzyme-inducing antiepileptics - these probably accelerate the metabolism of vitamin D. [21]
- L-Thyroxine over-replacement may contribute to osteoporosis, in a similar fashion as thyrotoxicosis does.[16] This can be relevant in subclinical hypothyroidism.
- Several drugs induce hypogonadism, for example aromatase inhibitors used in breast cancer, methotrexate and other anti-metabolite drugs, depot progesterone and gonadotropin-releasing hormone agonists.
- Anticoagulants - long-term use of heparin is associated with a decrease in bone density,[22] and warfarin (and related coumarins) have been linked with an increased risk in osteoporotic fracture in long-term use.[23]
- Proton pump inhibitors - these drugs inhibit the production of stomach acid; it is thought that this interferes with calcium absorption.[24] Chronic phosphate binding may also occur with aluminium-containing antacids.[16]
- Thiazolidinediones (used for diabetes) - rosiglitazone and possibly pioglitazone, inhibitors of PPARγ, have been linked with an increased risk of osteoporosis and fracture.[25]
- Chronic lithium therapy has been associated with osteoporosis.[16]
Diagnosis

The diagnosis of osteoporosis is made on measuring the bone mineral density (BMD). The most popular method is dual energy X-ray absorptiometry (DXA or DEXA). In addition to the detection of abnormal BMD, the diagnosis of osteoporosis requires investigations into potentially modifiable underlying causes; this may be done with blood tests and X-rays. Depending on the likelihood of an underlying problem, investigations for cancer with metastasis to the bone, multiple myeloma, Cushing's disease and other above mentioned causes may be performed.
Dual energy X-ray absorptiometry
Dual energy X-ray absorptiometry (DXA, formerly DEXA) is considered the gold standard for the diagnosis of osteoporosis. Osteoporosis is diagnosed when the bone mineral density is less than or equal to 2.5 standard deviations below that of a young adult reference population. This is translated as a T-score. The World Health Organization has established the following diagnostic guidelines:[1][10]
- T-score -1.0 or greater is "normal"
- T-score between -1.0 and -2.5 is "low bone mass" (or "osteopenia")
- T-score -2.5 or below is osteoporosis
When there has also been an osteoporotic fracture (also termed "low trauma-fracture" or "fragility fracture"), defined as one that occurs as a result of a fall from a standing height, the term "severe or established" osteoporosis is used.[1]
The International Society for Clinical Densitometry takes the position that a diagnosis of osteoporosis in men under 50 years of age should not be made on the basis of densitometric criteria alone. It also states that for pre-menopausal women, Z-scores (comparison with age group rather than peak bone mass) rather than T-scores should be used, and that the diagnosis of osteoporosis in such women also should not be made on the basis of densitometric criteria alone.[26]
Screening
The U.S. Preventive Services Task Force (USPSTF) recommended in 2002 that all women 65 years of age or older should be screened with bone densitometry.[27] The Task Force recommends screening only those women ages 60 to 64 years of age who are at increased risk. The best risk factor for indicating increased risk is lower body weight (weight < 70 kg), with less evidence for smoking or family history. There was insufficient evidence to make recommendations about the optimal intervals for repeated screening and the appropriate age to stop screening. Clinical prediction rules are available to guide selection of women ages 60-64 for screening. The Osteoporosis Risk Assessment Instrument (ORAI) may be the most sensitive strategy[28]
Regarding the screening of men, a cost-analysis study suggests that screening may be "cost-effective for men with a self-reported prior fracture beginning at age 65 years and for men 80 years and older with no prior fracture".[29] Also cost-effective is the screening of adult men from middle age on to detect any significant decrease in testosterone levels, say, below 300.
Pathogenesis
The underlying mechanism in all cases of osteoporosis is an imbalance between bone resorption and bone formation. In normal bone, there is constant matrix remodeling of bone; up to 10% of all bone mass may be undergoing remodeling at any point in time. The process takes place in bone multicellular units (BMUs) as first described by Frost in 1963.[30] Bone is resorbed by osteoclast cells (which derive from the bone marrow), after which new bone is deposited by osteoblast cells. [5]
The three main mechanisms by which osteoporosis develops are an inadequate peak bone mass (the skeleton develops insufficient mass and strength during growth), excessive bone resorption and inadequate formation of new bone during remodeling. An interplay of these three mechanisms underlies the development of fragile bone tissue.[5] Hormonal factors strongly determine the rate of bone resorption; lack of estrogen (e.g. as a result of menopause) increases bone resorption as well as decreasing the deposition of new bone that normally takes place in weight-bearing bones. The amount of estrogen needed to suppress this process is lower than that normally needed to stimulate the uterus and breast gland. The α-form of the estrogen receptor appears to be the most important in regulating bone turnover.[5] In addition to estrogen, calcium metabolism plays a significant role in bone turnover, and deficiency of calcium and vitamin D leads to impaired bone deposition; in addition, the parathyroid glands react to low calcium levels by secreting parathyroid hormone (parathormone, PTH), which increases bone resorption to ensure sufficient calcium in the blood. The role of calcitonin, a hormone generated by the thyroid that increases bone deposition, is less clear and probably not as significant as that of PTH.[5]
The activation of osteoclasts is regulated by various molecular signals, of which RANKL (receptor activator for nuclear factor κB ligand) is one of best studied. This molecule is produced by osteoblasts and other cells (e.g. lymphocytes), and stimulates RANK (receptor activator of nuclear factor κB). Osteoprotegerin (OPG) binds RANKL before it has an opportunity to bind to RANK, and hence suppresses its ability to increase bone resorption. RANKL, RANK and OPG are closely related to tumor necrosis factor and its receptors. The role of the wnt signalling pathway is recognized but less well understood. Local production of eicosanoids and interleukins is thought to participate in the regulation of bone turnover, and excess or reduced production of these mediators may underlie the development of osteoporosis.[5]
Trabecular bone is the sponge-like bone in the ends of long bones and vertebrae. Cortical bone is the hard outer shell of bones and the middle of long bones. Because osteoblasts and osteoclasts inhabit the surface of bones, trabecular bone is more active, more subject to bone turnover, to remodeling. Not only is bone density decreased, but the microarchitecture of bone is disrupted. The weaker spicules of trabecular bone break ("microcracks"), and are replaced by weaker bone. Common osteoporotic fracture sites, the wrist, the hip and the spine, have a relatively high trabecular bone to cortical bone ratio. These areas rely on trabecular bone for strength, and therefore the intense remodeling causes these areas to degenerate most when the remodeling is imbalanced.[citation needed]
Treatment
There are several alternatives of medication to treat osteoporosis, depending on gender, though lifestyle changes are also very frequently an aspect of treatment.
Medication
Bisphosphonates are the main pharmacological measures for treatment. However, newer drugs have appeared in the 1990s, such as teriparatide and strontium ranelate.
- Bisphosphonates
In confirmed osteoporosis, bisphosphonate drugs are the first-line treatment in women. The most often prescribed bisphosphonates are presently sodium alendronate (Fosamax) 10 mg a day or 70 mg once a week, risedronate (Actonel) 5 mg a day or 35 mg once a week and or ibandronate (Boniva) once a month.
A 2007 manufacturer-supported study suggested that in patients who had suffered a low-impact hip fracture, annual infusion of 5 mg zoledronic acid reduced risk of any fracture by 35% (from 13.9 to 8.6%), vertebral fracture risk from 3.8% to 1.7% and non-vertebral fracture risk from 10.7% to 7.6%. This study also found a mortality benefit: after 1.9 years, 9.6% of the study group (as opposed to 13.3% of the control group) had died of any cause, indicating a mortality benefit of 28%.[31]
Oral bisphosphonates are relatively poorly absorbed, and must therefore be taken on an empty stomach, with no food or drink to follow for the next 30 minutes. They are associated with esophagitis and are therefore sometimes poorly tolerated; weekly or monthly administration (depending on the preparation) decreases likelihood of esophagitis, and is now standard. Although intermittent dosing with the intravenous formulations such as zolendronate avoids oral tolerance problems, these agents are implicated at higher rates in a rare but unpleasant mouth disease called osteonecrosis of the jaw.[32] For this reason, oral bisphosphonate therapy is probably to be preferred, and prescribing advice now recommends any remedial dental work to be carried out prior to commencing treatment.[33]
- Teriparatide
Recently, teriparatide (Forteo, recombinant parathyroid hormone residues 1–34) has been shown to be effective in osteoporosis. It acts like parathyroid hormone and stimulates osteoblasts, thus increasing their activity. It is used mostly for patients with established osteoporosis (who have already fractured), have particularly low BMD or several risk factors for fracture or cannot tolerate the oral bisphosphonates. It is given as a daily injection with the use of a pen-type injection device. Teriparatide is only licensed for treatment if bisphosphonates have failed or are contraindicated (however, this differs by country and is not required by the FDA in the USA. However, patients with previous radiation therapy, or Paget's disease, or young patients should avoid this medication).
- Strontium ranelate
Oral strontium ranelate is an alternative oral treatment, belonging to a class of drugs called "dual action bone agents" (DABAs) by its manufacturer. It has proven efficacy, especially in the prevention of vertebral fracture.[34] In laboratory experiments, strontium ranelate was noted to stimulate the proliferation of osteoblasts, as well as inhibiting the proliferation of osteoclasts.
Strontium ranelate is taken as a 2 g oral suspension daily, and is licenced for the treatment of osteoporosis to prevent vertebral and hip fracture. Strontium ranelate has side effect benefits over the bisphosphonates, as it does not cause any form of upper GI side effect, which is the most common cause for medication withdrawal in osteoporosis. In studies a small increase in the risk of venous thromboembolism was noted,[35] the cause for which has not been determined. This suggests it may be less suitable in patients at risk for thrombosis for different reasons. The uptake of (heavier) strontium in place of calcium into bone matrix results in a substantial and disproportionate increase in bone mineral density as measured on DXA scanning[36], making further followup of bone density by this method harder to interpret for strontium treated patients. A correction algorithm has been devised.[37]
Although strontium ranelate is effective, it's not approved for use in the United States yet. However, strontium citrate is available in the U.S. from several well-known vitamin manufacturers. Most researchers believe that strontium is safe and effective no matter what form it's used. The ranelate form is simply a device invented by the Servier company of France so that they could patent their version of strontium.[citation needed]
Strontium, no matter what the form, must be water-soluble and ionized in the stomach acid. Stontium is then protein-bound for transport from the intestinal tract into the blood stream. Unlike drugs like sodium alendronate (Fosamax), strontium doesn't inhibit bone recycling and, in fact, may produce stronger bones. Studies have shown that after five years alendronate may even cause bone loss, while strontium continues to build bone during lifetime use.[citation needed]
Strontium must not be taken with food or calcium-containing preparations as calcium competes with strontium during uptake. However, it's essential that calcium, magnesium, and vitamin D in theraputic amounts must be taken daily, but not at the same time as strontium. Strontium should be taken on an empty stomach at night.[citation needed]
- Hormone replacement
Estrogen replacement therapy remains a good treatment for prevention of osteoporosis but, at this time, is not recommended unless there are other indications for its use as well. There is uncertainty and controversy about whether estrogen should be recommended in women in the first decade after the menopause.
In hypogonadal men testosterone has been shown to give improvement in bone quantity and quality, but, as of 2008, there are no studies of the effects on fractures or in men with a normal testosterone level.[18]
- Selective estrogen receptor modulator (SERM)
SERMs are a class of medications that act on the estrogen receptors throughout the body in a selective manner. Normally, bone mineral density (BMD) is tightly regulated by a balanace between osteoblast and osteoclast activity in the trabecular bone. Estrogen has a major role in regulation of the bone formation-resorption equilibrium, as it stimulates osteoblast activity. Some SERMs such as raloxifene (Evista), act on the bone by slowing bone resorption by the osteoclasts.[38] SERMs have been proved as effective in clinical trials.[39][40]
Nutrition
- Calcium
Calcium is required to support bone growth, bone healing and maintain bone strength and is one aspect of treatment for osteoporosis. Recommendations for calcium intake vary depending country and age; for individuals at higher risk of osteoporosis (after fifty years of age) the amount recommended by US health agencies is 1,200 mg per day. Calcium supplements can be used to increase dietary intake, and absorption is optimized through taking in several small (500 mg or less) doses throughout the day.[41] The role of calcium in preventing and treating osteoporosis is unclear - some populations with extremely low calcium intake also have extremely low rates of bone fracture, and others with high rates of calcium intake through milk and milk products have higher rates of bone fracture. Other factors, such as protein, salt and vitamin D intake, exercise and exposure to sunlight, can all influence bone mineralization, making calcium intake one factor among many in the development of osteoporosis.[42]
A meta-analysis of randomized controlled trials involving calcium and calcium plus vitamin D supported the use of high levels of calcium (1,200 mg or more) and vitamin D (800 IU or more), though outcomes varied depending on which measure was used to assess bone health (rates of fracture versus rates of bone loss).[43] The meta-analysis, along with another study, also supported much better outcomes for patients with high compliance to the treatment protocol.[44] In contrast, despite earlier reports in improved high density lipoprotein (HDL, "good cholesterol") in calcium supplementation, a possible increase in the rate of myocardial infarction (heart attack) was found in a study in New Zealand in which 1471 women participated. If confirmed, this would indicate that calcium supplementation in women otherwise at low risk of fracture may cause more harm than good.[45]
- Vitamin D
Some studies have shown that a high intake of vitamin D reduces fractures in the elderly,[43][46] though the Women's Health Initiative found that though calcium plus vitamin D did increase bone density, it did not affect hip fracture but did increase formation of kidney stones.[47]
Exercise
Multiple studies have shown that aerobics, weight bearing, and resistance exercises can all maintain or increase BMD in postmenopausal women.[48] Many researchers have attempted to pinpoint which types of exercise are most effective at improving BMD and other metrics of bone quality, however results have varied. One year of regular jumping exercises appears to increase the BMD and moment of inertia of the proximal tibia[49] in normal postmenopausal women. Treadmill walking, gymnastic training, stepping, jumping, endurance, and strength exercises all resulted in significant increases of L2-L4 BMD in osteopenic postmenopausal women.[50][51][52] Strength training elicited improvements specifically in distal radius and hip BMD.[53] Exercise combined with other pharmacological treatments such as hormone replacement therapy (HRT) has been shown to increases BMD more than HRT alone.[54]
Additional benefits for osteoporotic patients other than BMD increase include improvements in balance, gait, and a reduction in risk of falls.[55]
Prognosis
| WHO category | Age 50-64 | Age > 64 | Overall |
|---|---|---|---|
| Normal | 5.3 | 9.4 | 6.6 |
| Osteopenia | 11.4 | 19.6 | 15.7 |
| Osteoporosis | 22.4 | 46.6 | 40.6 |
Although osteoporosis patients have an increased mortality rate due to the complications of fracture, most patients die with the disease rather than of it.
Hip fractures can lead to decreased mobility and an additional risk of numerous complications (such as deep venous thrombosis and/or pulmonary embolism, pneumonia). The 6-month mortality rate following hip fracture is approximately 13.5%, and a substantial proportion (almost 13%) of people who have suffered a hip fracture need total assistance to mobilize after a hip fracture.[57]
Vertebral fractures, while having a smaller impact on mortality, can lead to severe chronic pain of neurogenic origin, which can be hard to control, as well as deformity. Though rare, multiple vertebral fractures can lead to such severe hunch back (kyphosis) that the resulting pressure on internal organs can impair one's ability to breathe.
Apart from risk of death and other complications, osteoporotic fractures are associated with a reduced health-related quality of life.[58]
Epidemiology
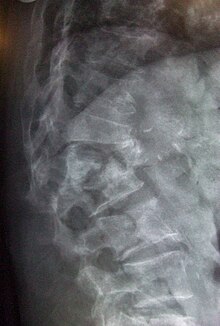
It is estimated[citation needed] that 1 in 3 women and 1 in 12 men over the age of 50 worldwide have osteoporosis. It is responsible for millions of fractures annually, mostly involving the lumbar vertebrae, hip, and wrist. Fragility fractures of ribs are also common in men.
Hip fractures
Hip fractures are responsible for the most serious consequences of osteoporosis. In the United States, more than 250,000 hip fractures annually are attributible to Osteoporosis.[59] It is estimated that a 50-year-old white woman has a 17.5% lifetime risk of fracture of the proximal femur. The incidence of hip fractures increases each decade from the sixth through the ninth for both women and men for all populations. The highest incidence is found among those men and women ages 80 or older.[60]
Vertebral fractures
Between 35-50% of all women over 50 had at least one vertebral fracture. In the United States, 700,000 vertebral fractures occur annually, but only about a third are recognized. In a series of 9704 of women aged 68.8 on average studied for 15 years, 324 had already suffered a vertebral fracture at entry into the study; 18.2% developed a vertebral fracture, but that risk rose to 41.4% in women who had a previous vertebral fracture.[61]
Wrist
In the United States, 250,000 wrist fractures annually are attributable to Osteoporosis.[59] Wrist fractures are the third most common type of osteoporotic fractures. The lifetime risk of sustaining a Colles' fracture is about 16% for white women. By the time women reach age 70, about 20% have had at least one wrist fracture.[60]
Rib Fractures
Fragility fractures of the ribs are common in men as young as age thirty-five on. These are often overlooked as signs of osteoporosis as these men are often physically active and suffer the fracture in the course of physical activity. An example would be as a result of falling while water skiing or jet skiing. However, a quick test of the individual's testosterone level following the diagnosis of the fracture will readily reveal whether that individual might be at risk.
Prevention
Methods to prevent osteoporosis include changes of lifestyle. However, there are medications that can be used for prevention as well. As a different concept there are osteoporosis ortheses which help to prevent spine fractions and support the building up of muscles. Fall prevention can help prevent osteoporosis complications.
Lifestyle
Lifestyle prevention of osteoporosis is in many aspects inversions from potentially modifiable risk factors. As tobacco smoking and unsafe alcohol intake have been linked with osteoporosis, smoking cessation and moderation of alcohol intake are commonly recommended in the prevention of osteoporosis.[citation needed]
- Exercise
Achieving a higher peak bone mass through exercise and proper nutrition during adolescence is important for the prevention of osteoporosis. Exercise and nutrition throughout the rest of the life delays bone degeneration. Jogging, walking, or stair climbing at 70-90% of maximum effort three times per week, along with 1,500 mg of calcium per day, increased bone density of the lumbar (lower) spine by 5% over 9 months. Individuals already diagnosed with osteopenia or osteoporosis should discuss their exercise program with their physician to avoid fractures.[62]
- Nutrition
A proper nutrition is a diet sufficient in calcium and vitamin D. Patients at risk for osteoporosis (e.g. steroid use) are generally treated with vitamin D and calcium supplements and often with bisphosphonates. In renal disease, more active forms of Vitamin D such as paracalcitol or (1,25-dihydroxycholecalciferol or calcitriol which is the main biologically active form of vitamin D) is used, as the kidney cannot adequately generate calcitriol from calcidiol (25-hydroxycholecalciferol) which is the storage form of vitamin D.
High dietary protein intake increases calcium excretion in urine and has been linked to increased risk of fractures in research studies.[63] Other investigations have shown that protein is required for calcium absorption, but that excessive protein consumption inhibits this process. No interventional trials have been performed on dietary protein in the prevention and treatment of osteoporosis.[64]
Medication
Just as for treatment, bisphosphonate can be used in cases of very high risk. Other medicines prescribed for prevention of osteoporosis include raloxifene (Evista), a selective estrogen receptor modulator (SERM).
Estrogen replacement therapy remains a good treatment for prevention of osteoporosis but, at this time, is not recommended unless there are other indications for its use as well. There is uncertainty and controversy about whether estrogen should be recommended in women in the first decade after the menopause.
In hypogonadal men testosterone has been shown to give improvement in bone quantity and quality, but, as of 2008, there are no studies of the effects on fractures or in men with a normal testosterone level.[18]
History
The link between age-related reductions in bone density and fracture risk goes back at least to Astley Cooper, and the term "osteoporosis" and recognition of its pathological appearance is generally attributed to the French pathologist Jean Lobstein.[65] The American endocrinolgist Fuller Albright linked osteoporosis with the postmenopausal state.[66] Bisphosponates, which revolutionized the treatment of osteoporosis, were discovered in the 1960s.[67]
See also
- Back pain
- Hip protector
- Osteoporosis Orthesis
- Dental X-ray
- Osteopetrosis
- Osteoimmunology
- Ovariectomized rat model for osteoporosis research
References
- ^ a b c WHO (1994). "Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group". World Health Organization technical report series. 843: 1–129. PMID 7941614.
- ^ Kim DH, Vaccaro AR (2006). "Osteoporotic compression fractures of the spine; current options and considerations for treatment". The spine journal : official journal of the North American Spine Society. 6 (5): 479–87. doi:10.1016/j.spinee.2006.04.013. PMID 16934715.
- ^ Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ (2007). "Will my patient fall?". JAMA. 297 (1): 77–86. doi:10.1001/jama.297.1.77. PMID 17200478.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Melton LJ (2003). "Epidemiology worldwide". Endocrinol. Metab. Clin. North Am. 32 (1): 1–13, v. PMID 12699289.
- ^ a b c d e f Raisz L (2005). "Pathogenesis of osteoporosis: concepts, conflicts, and prospects". J Clin Invest. 115 (12): 3318–25. doi:10.1172/JCI27071. PMID 16322775.
- ^ Ojo F, Al Snih S, Ray LA, Raji MA, Markides KS (2007). "History of fractures as predictor of subsequent hip and nonhip fractures among older Mexican Americans". Journal of the National Medical Association. 99 (4): 412–8. PMID 17444431.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Poole KE, Compston JE (2006). "Osteoporosis and its management". BMJ. 333 (7581): 1251–6. doi:10.1136/bmj.39050.597350.47. PMID 17170416.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Berg KM, Kunins HV, Jackson JL; et al. (2008). "Association between alcohol consumption and both osteoporotic fracture and bone density". Am J Med. 121 (5): 406–18. doi:10.1016/j.amjmed.2007.12.012.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b Nieves JW (2005). "Osteoporosis: the role of micronutrients". Am J Clin Nutr. 81 (5): 1232S–1239S. PMID 15883457.
- ^ a b c d e f g h i j k l m n WHO Scientific Group on the Prevention and Management of Osteoporosis (2000 : Geneva, Switzerland) (2003). "Prevention and management of osteoporosis : report of a WHO scientific group" (pdf). Retrieved 2007-05-31.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^ Wong PK, Christie JJ, Wark JD (2007). "The effects of smoking on bone health". Clin. Sci. 113 (5): 233–41. doi:10.1042/CS20060173. PMID 17663660.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shapses SA, Riedt CS (2006). "Bone, body weight, and weight reduction: what are the concerns?". J. Nutr. 136 (6): 1453–6. PMID 16702302.
- ^ Staessen J, Roels H, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, Fagard R (1999). "Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group". Lancet. 353 (9159): 1140–4. doi:10.1016/S0140-6736(98)09356-8. PMID 10209978.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Tucker KL, Morita K, Qiao N, Hannan MT, Cupples LA, Kiel DP (2006). "Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study". Am. J. Clin. Nutr. 84 (4): 936–42. PMID 17023723.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Soft drinks in schools". Pediatrics. 113 (1 Pt 1): 152–4. 2004. PMID 14702469.
- ^ a b c d e Simonelli, C; et al. (2006). "ICSI Health Care Guideline: Diagnosis and Treatment of Osteoporosis, 5th edition" (PDF). Institute for Clinical Systems Improvement. Retrieved 2008-04-08.
{{cite web}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) - ^ a b c d e f g h i j k l Kohlmeier, Lynn Kohlmeier (1998). "Osteoporosis - Risk Factors, Screening, and Treatment". Medscape Portals. Retrieved 2008-05-11.
- ^ a b c d Ebeling PR (2008). "Clinical practice. Osteoporosis in men". N Engl J Med. 358 (14): 1474–82. doi:10.1056/NEJMcp0707217. PMID 18385499.
- ^ Bone and Tooth Society of Great Britain, National Osteoporosis Society, Royal College of Physicians (2003). Glucocorticoid-induced Osteoporosis (PDF). London, UK: Royal College of Physicians of London. ISBN 1-860-16173-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Gourlay M, Franceschini N, Sheyn Y (2007). "Prevention and treatment strategies for glucocorticoid-induced osteoporotic fractures". Clin Rheumatol. 26 (2): 144–53. doi:10.1007/s10067-006-0315-1. PMID 16670825.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Petty SJ, O'Brien TJ, Wark JD (2007). "Anti-epileptic medication and bone health". Osteoporosis international. 18 (2): 129–42. doi:10.1007/s00198-006-0185-z. PMID 17091219.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ruiz-Irastorza G, Khamashta MA, Hughes GR (2002). "Heparin and osteoporosis during pregnancy: 2002 update". Lupus. 11 (10): 680–2. doi:10.1191/0961203302lu262oa. PMID 12413068.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gage BF, Birman-Deych E, Radford MJ, Nilasena DS, Binder EF (2006). "Risk of osteoporotic fracture in elderly patients taking warfarin: results from the National Registry of Atrial Fibrillation 2". Arch. Intern. Med. 166 (2): 241–6. doi:10.1001/archinte.166.2.241. PMID 16432096.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yang YX, Lewis JD, Epstein S, Metz DC (2006). "Long-term proton pump inhibitor therapy and risk of hip fracture". JAMA. 296: 2947–53. doi:10.1001/jama.296.24.2947. PMID 17190895.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Murphy CE, Rodgers PT (2007). "Effects of thiazolidinediones on bone loss and fracture". Ann Pharmacother. 41 (12): 2014–8. doi:10.1345/aph.1K286. PMID 17940125.
- ^ Leib ES, Lewiecki EM, Binkley N, Hamdy RC (2004). "Official positions of the International Society for Clinical Densitometry". J Clin Densitom. 7 (1): 1799. doi:10.1385/JCD:7:1:1. PMID 14742881.
{{cite journal}}: CS1 maint: multiple names: authors list (link) quoted in: "Diagnosis of osteoporosis in men, premenopausal women, and children" - ^ U.S. Preventive Services Task Force (2002). "Screening for osteoporosis in postmenopausal women: recommendations and rationale". Ann. Intern. Med. 137 (6): 526–8. PMID 12230355.
- ^ Martínez-Aguilà D, Gómez-Vaquero C, Rozadilla A, Romera M, Narváez J, Nolla JM (2007). "Decision rules for selecting women for bone mineral density testing: application in postmenopausal women referred to a bone densitometry unit". J. Rheumatol. 34 (6): 1307–12. PMID 17552058.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schousboe JT, Taylor BC, Fink HA; et al. (2007). "Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men". JAMA. 298 (6): 629–37. doi:10.1001/jama.298.6.629. PMID 17684185.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Frost HM, Thomas CC. Bone Remodeling Dynamics. Springfield, IL: 1963.
- ^ Lyles KW, Colón-Emeric CS, Magaziner JS; et al. (2007). "Zoledronic acid and clinical fractures and mortality after hip fracture". N Engl J Med. 357: published online 2007–09–17. doi:10.1056/NEJMoa074941. PMID 17878149.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Purcell, P. Boyd, I (2005). "Bisphosphonates and osteonecrosis of the jaw". Medical Journal of Australia. 182 (8): 417–418.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "6.6.2 Bisphosphonates". British National Formulary (54 ed.). British Medical Association and Royal Pharmaceutical Society of Great Britain. 2007. pp. p403.
{{cite book}}:|pages=has extra text (help); Unknown parameter|month=ignored (help) - ^ Meunier PJ, Roux C, Seeman E; et al. (2004). "The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis". N. Engl. J. Med. 350 (5): 459–68. doi:10.1056/NEJMoa022436. PMID 14749454.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ O'Donnell S, Cranney A, Wells GA, Adachi JD, Reginster JY (2006). "Strontium ranelate for preventing and treating postmenopausal osteoporosis". Cochrane database of systematic reviews (Online) (4): CD005326. doi:10.1002/14651858.CD005326.pub3. PMID 17054253.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Reginster JY, Seeman E, De Vernejoul MC; et al. (2005). "Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: treatment of peripheral osteoporosis (TROPOS) study". J Clin Endorinol Metab. 90: 2816–22. PMID 15728210.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Blake GM, Fogelman I (2007). "The correction of BMD measurements for bone strontium content". J Clin Densitom. 10 (3): 259–65. doi:10.1016/j.jocd.2007.03.102. PMID 17543560.
- ^ Taranta A, Brama M, Teti A; et al. (2002). "The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro". Bone. 30 (2): 368–76. PMID 11856644.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Meunier PJ, Vignot E, Garnero P; et al. (1999). "Treatment of postmenopausal women with osteoporosis or low bone density with raloxifene. Raloxifene Study Group". Osteoporos Int. 10 (4): 330–6. PMID 10692984.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Meunier PJ, Vignot E, Garnero P; et al. (1999). "Treatment of postmenopausal women with osteoporosis or low bone density with raloxifene. Raloxifene Study Group". Osteoporos Int. 10 (4): 330–6. PMID 10692984.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "Nutrition and Bone Health". NIAMS. 2005-11-01. Retrieved 2008-01-28.
- ^ "Calcium & Milk". Harvard School of Public Health. 2007. Retrieved 2008-01-28.
- ^ a b Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A (2007). "Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis". Lancet. 370 (9588): 657–66. doi:10.1016/S0140-6736(07)61342-7. PMID 17720017.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Prince RL, Devine A, Dhaliwal SS, Dick IM (2006). "Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women". Arch. Intern. Med. 166 (8): 869–75. doi:10.1001/archinte.166.8.869. PMID 16636212.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bolland MJ, Barber PA, Doughty RN; et al. (2008). "Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial". BMJ. 336: 262. doi:10.1136/bmj.39440.525752.BE. PMID 18198394.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B (2005). "Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials". JAMA. 293 (18): 2257–64. doi:10.1001/jama.293.18.2257. PMID 15886381.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jackson RD, LaCroix AZ, Gass M; et al. (2006). "Calcium plus vitamin D supplementation and the risk of fractures". N. Engl. J. Med. 354 (7): 669–83. doi:10.1056/NEJMoa055218. PMID 16481635.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Bonaiuti D, Shea B, Iovine R; et al. (2002). "Exercise for preventing and treating osteoporosis in postmenopausal women". Cochrane database of systematic reviews (Online) (3): CD000333. doi:10.1002/14651858.CD000333. PMID 12137611.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Cheng S, Sipilä S, Taaffe DR, Puolakka J, Suominen H (2002). "Change in bone mass distribution induced by hormone replacement therapy and high-impact physical exercise in post-menopausal women". Bone. 31 (1): 126–35. doi:10.1016/S8756-3282(02)00794-9. PMID 12110425.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chien MY, Wu YT, Hsu AT, Yang RS, Lai JS (2000). "Efficacy of a 24-week aerobic exercise program for osteopenic postmenopausal women". Calcif. Tissue Int. 67 (6): 443–8. doi:10.1007/s002230001180. PMID 11289692.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Iwamoto J, Takeda T, Ichimura S (2001). "Effect of exercise training and detraining on bone mineral density in postmenopausal women with osteoporosis". Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association. 6 (2): 128–32. doi:10.1007/s0077610060128. PMID 11484097.
{{cite journal}}: Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Kemmler W, Engelke K, Weineck J, Hensen J, Kalender WA (2003). "The Erlangen Fitness Osteoporosis Prevention Study: a controlled exercise trial in early postmenopausal women with low bone density-first-year results". Archives of physical medicine and rehabilitation. 84 (5): 673–82. PMID 12736880.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kerr D, Morton A, Dick I, Prince R (1996). "Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent". J. Bone Miner. Res. 11 (2): 218–25. PMID 8822346.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Villareal DT, Binder EF, Yarasheski KE; et al. (2003). "Effects of exercise training added to ongoing hormone replacement therapy on bone mineral density in frail elderly women". J Am Geriatr Soc. 51 (7): 985–90. doi:10.1046/j.1365-2389.2003.51312.x. PMID 12834519.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR (2005). "Significant reduction in risk of falls and back pain in osteoporotic-kyphotic women through a Spinal Proprioceptive Extension Exercise Dynamic (SPEED) program". Mayo Clin Proc. 80 (7): 849–55. PMID 16007888.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD (2007). "Low bone mineral density and fracture burden in postmenopausal women". CMAJ. 177 (6): 575–80. doi:10.1503/cmaj.070234. PMID 17846439.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hannan EL, Magaziner J, Wang JJ; et al. (2001). "Mortality and locomotion 6 months after hospitalization for hip fracture: risk factors and risk-adjusted hospital outcomes". JAMA. 285 (21): 2736–42. doi:10.1001/jama.285.21.2736. PMID 11386929.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Brenneman SK, Barrett-Connor E, Sajjan S, Markson LE, Siris ES (2006). "Impact of recent fracture on health-related quality of life in postmenopausal women". J. Bone Miner. Res. 21 (6): 809–16. doi:10.1359/jbmr.060301. PMID 16753011.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Riggs, B.L.; Melton, Lj 3.r.d. (2005). "The worldwide problem of osteoporosis: insights afforded by epidemiology". Bone. PMID 8573428.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ a b "MerckMedicus Modules: Osteoporosis - Epidemiology". Merck & Co., Inc. Retrieved 2008-06-13.
- ^ Cauley JA, Hochberg MC, Lui LY; et al. (2007). "Long-term Risk of Incident Vertebral Fractures". JAMA. 298: 2761–2767. doi:10.1001/jama.298.23.2761. PMID 18165669.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Dalsky GP, Stocke KS, Ehsani AA, Slatopolsky E, Lee WC, Birge SJ (1988). "Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women". Ann. Intern. Med. 108 (6): 824–8. PMID 3259410.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Feskanich D, Willett WC, Stampfer MJ, Colditz GA (1996). "Protein consumption and bone fractures in women". Am. J. Epidemiol. 143 (5): 472–9. PMID 8610662.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kerstetter JE, O'Brien KO, Insogna KL (2003). "Dietary protein, calcium metabolism, and skeletal homeostasis revisited". Am. J. Clin. Nutr. 78 (3 Suppl): 584S–592S. PMID 12936953.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lobstein JGCFM. Lehrbuch der pathologischen Anatomie. Stuttgart: Bd II, 1835.
- ^ Albright F, Bloomberg E, Smith PH (1940). "Postmenopausal osteoporosis". Trans. Assoc. Am. Physicians. 55: 298–305.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - ^ Patlak M (2001). "Bone builders: the discoveries behind preventing and treating osteoporosis". FASEB J. 15 (10): 1677E–E. doi:10.1096/fj.15.10.1677e. PMID 11481214.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
- Osteoporosis at Curlie
- Osteoporosis risk assessment tools
- Diet, Nutrition and the prevention of osteoporosis the World Health Organization and Food and Agriculture Organization (2003)
- Bone Health and Osteoporosis: A Report of the Surgeon General distributed by the U.S. Department of Health and Human Services
- NOF.org The National Osteoporosis Foundation
