α-mannosidosis
| Classification according to ICD-10 | |
|---|---|
| E77.1 | Defects in glycoprotein degradation mannosidosis |
| ICD-10 online (WHO version 2019) | |
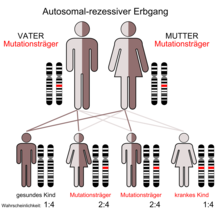
The α-Mannosidosis is a very rare autosomal - recessive inherited lysosomal storage disease .
Prevalence
With a prevalence of around 1: 500,000 in live-born children, α-mannosidosis is an extremely rare disease.
Symptoms
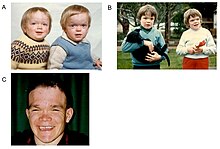
In affected patients, α-mannosidosis is characterized by immunodeficiency , facial abnormalities, changes in the skeleton , hearing loss and intellectual deficits. The first symptoms can already be visible at birth. With increasing age, however, the disease progresses and the condition of those affected continues to worsen. In addition to the symptoms already mentioned, some may be born with clubfoot or develop hydrocephalus (water head) in the first twelve months .
The immune deficiency causes recurrent infections , especially in the first decade of life . The skeletal anomalies include mild to moderate dysostosis multiplex , scoliosis (lateral bending of the spine) and deformation of the sternum . The facial abnormalities include an enlarged skull, prominent forehead, rounded eyebrows, saddle nose , macroglossia (enlarged tongue), widely spaced teeth, and progeny (a type of jaw misalignment). Slight squint is also a common symptom.
The hearing loss is characterized by a moderate to severe disturbance of the sound perception. Patients' motor skills are also affected by muscle weakness , joint abnormalities, and ataxia .
Symptoms can vary significantly from patient to patient.
Genetics and Pathogenesis
Alpha-mannosidosis is based on an autosomal recessive inheritance . Mutations in MAN2B1 - gene , located on 19 chromosome gene locus P13.2-q12 is, are the cause of the disease. The MAN2B1 gene codes for the enzyme α-mannosidase . Mutations in this gene product can cause a reduced activity of α-mannosidase, as a result of which high-mannose glycoconjugates accumulate in the tissue of the affected patient .
diagnosis
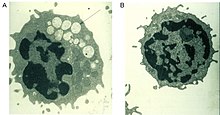
The diagnosis can be made by determining the activity of α-mannosidase in leukocytes or other nucleated cells. A DNA analysis ('genetic test') can be performed to confirm the results. The excretion of increased amounts of high-mannose oligosaccharides in the urine is an indication of the disease, but not specific evidence. A prenatal diagnosis is possible both biochemically and molecularly genetically.
therapy
So far there is no established curative therapy .
Enzyme replacement therapy
One promising therapeutic approach is the enzyme replacement therapy (ERT or EET of Engl. Enzyme Replacement Therapy ).
Research work
In the animal model of guinea pigs , EET was able to reduce the accumulation of oligosaccharides in the tissue. The brain was an exception, as the applied enzyme can not reach the brain due to the blood-brain barrier . Similar experiments with knockout mice - here was Man2b1 - gene of mice off - led, surprisingly, to a decrease of the oligosaccharides in the brain of experimental animals. Establishing the ERT for patients with α-mannosidosis was one of the goals of the European Hue-Man project initiated in 2006 .
drug
On March 23, 2018, Velmanase alfa (trade name Lamzede from Chiesi ) was approved as an enzyme replacement therapy for the treatment of non-neurological manifestations in patients with mild to moderate alpha-mannosidosis in the European Union .
Velmanase alfa is a recombinant form of human alpha-mannosidase. It is said to supplement or replace the natural alpha-mannosidase. The medicine is given as an intravenous infusion once a week. A clinical study found evidence that velmanase alfa can slow the progression of an existing disease.
Allogeneic stem cell transplant
Some patients have had an allogeneic stem cell transplant . Some of the results were very promising. However, the therapeutic benefit must be carefully weighed against the considerable risks of allogeneic stem cell transplantation. Allogeneic stem cell transplantation is a therapeutic option, especially in young patients in the first decade of life, with less advanced disease.
Zinc substitution
The administration of zinc sulfate (zinc substitution) showed a significant increase in the activity of α-mannosidase in vitro , which is why this was initially a widespread therapeutic approach. However, in long-term studies there was no significant effect in patients with α-mannosidosis.
Other therapy
The usual therapy is purely symptomatic. Ideally, proactive treatment, such as physiotherapy , which prevents possible future complications, is used. The infections caused by the immune deficiency often have to be treated.
forecast
The patient's condition worsens with age. The function of the skeletal muscles and motor skills are increasingly decreasing, which means that the majority of those affected are wheelchair-bound. No patient is completely socially independent. Many patients live to be older than 50 years. As the disease progresses, all patients become hard of hearing and need a hearing aid.
Initial description
In 1967, the doctor Per-Arne Öckerman (* 1933) from Lund University in Sweden was the first to describe a new form of lysosomal storage disease in a boy with symptoms similar to Hurler's syndrome , but in which no mucopolysaccharides are accumulated.
Veterinary medicine
In cattle , especially the ' Aberdeen Angus ' breed, α-mannosidosis is a relatively common disease.
literature
- KW Moremen: Golgi alpha-mannosidase II deficiency in vertebrate systems: implications for asparagine-linked oligosaccharide processing in mammals. In: Biochimica et Biophysica Acta . Volume 1573, Number 3, December 2002, pp. 225-235, PMID 12417404 .
- T. Beccari et al .: Lysosomal alpha-D-mannosidase. In: Bioscience Reports . Volume 19, Number 3, June 1999, pp. 157-162, PMID 10513892 .
- JP Kistler et al .: Mannosidosis. New clinical presentation, enzyme studied, and carbohydrate analysis. In: Archives of Neurology . Volume 34, Number 1, January 1977, pp. 45-51, PMID 12732 .
- Y. Gotoda et al .: Missense and nonsense mutations in the lysosomal alpha-mannosidase gene (MANB) in severe and mild forms of alpha-mannosidosis. In: American Journal of Human Genetics . Volume 63, Number 4, October 1998, pp. 1015-1024, doi : 10.1086 / 302048 . PMID 9758606 . PMC 1377481 (free full text).
- A. Gutschalk et al .: Adult alpha-mannosidosis: clinical progression in the absence of demyelination. In: Neurology . Volume 63, Number 9, November 2004, pp. 1744-1746, PMID 15534274 .
Web links
- Α-mannosidosis. In: Online Mendelian Inheritance in Man . (English)
- Α-mannosidosis. In: Orphanet (Rare Disease Database).
Individual evidence
- ↑ a b c d e f g h i j k D. Malm and O. Nilsson: Alpha-mannosidosis. In: Orphanet Journal of Rare Diseases 3, 2008, 21. (Review, Open Access , CC-by-2.0 )
- ^ AC Crawley et al .: Enzyme replacement therapy in alpha-mannosidosis guinea-pigs. In: Molecular Genetics and Metabolism. Volume 89, number 1-2, 2006 Sep-Oct, pp. 48-57, doi : 10.1016 / j.ymgme.2006.05.005 . PMID 16807033 .
- ↑ DP Roces, et al .: Efficacy of enzyme replacement therapy in alpha-mannosidosis mice: a preclinical animal study. In: Human Molecular Genetics . Volume 13, Number 18, September 2004, pp. 1979-1988, doi : 10.1093 / hmg / ddh220 . PMID 15269179 .
- ^ Towards The Development Of An Effective Enzyme Replacement Therapy For Human Alpha-Mannosidosis. Retrieved November 7, 2009
- ↑ European Medicines Agency - Find medicine - Lamzede. Retrieved July 4, 2018 .
- ↑ Lamzede - information for professionals and instructions for use. Retrieved July 4, 2018 .
- ↑ SS Grewal et al .: Effective treatment of alpha-mannosidosis by allogeneic hematopoietic stem cell transplantation. In: The Journal of Pediatrics . Volume 144, Number 5, May 2004, pp. 569-573, doi : 10.1016 / j.jpeds.2004.01.025 . PMID 15126988 .
- ^ DA Wall, DK Grange, P. Goulding, M. Daines, A. Luisiri, S. Kotagal: Bone marrow transplantation for the treatment of alpha-mannosidosis. In: The Journal of pediatrics. Volume 133, Number 2, August 1998, pp. 282-285, PMID 9709723 .
- ^ A. Will et al .: Bone marrow transplantation in the treatment of alpha-mannosidosis. In: Archives of Disease in Childhood . Volume 62, Number 10, October 1987, pp. 1044-1049, PMID 3314721 . PMC 1778651 (free full text).
- ^ SU Walkley et al .: Bone marrow transplantation corrects the enzyme defect in neurons of the central nervous system in a lysosomal storage disease. In: Proceedings of the National Academy of Sciences . Volume 91, Number 8, April 1994, pp. 2970-2974, PMID 8159689 . PMC 43496 (free full text).
- ^ LT Wong et al .: Oral zinc therapy in the treatment of alpha-mannosidosis. In: American journal of medical genetics. Volume 46, Number 4, June 1993, pp. 410-414, doi : 10.1002 / ajmg.1320460413 . PMID 8357013 .
- ↑ PA Öckerman: A generalized storage disorder resembling Hurler's syndrome. In: The Lancet 2, 1967, doi : 10.1016 / S0140-6736 (67) 92303-3 . P. 239.
- ↑ JD Hocking et al .: Deficiency of alpha-mannosidase in Angus cattle. An inherited lysosomal storage disease. In: The Biochemical journal. Volume 128, Number 1, June 1972, pp. 69-78, PMID 4673577 . PMC 1173571 (free full text).
- ^ HW Leipold et al .: Mannosidosis of Angus calves. In: Journal of the American Veterinary Medical Association. Volume 175, Number 5, September 1979, pp. 457-459, PMID 500478 .