1,3,5-triphenylbenzene
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
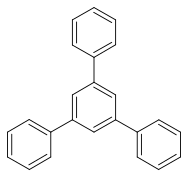
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 1,3,5-triphenylbenzene | ||||||||||||||||||
| Molecular formula | C 24 H 18 | ||||||||||||||||||
| Brief description |
light brown solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 306.40 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.199 g cm −3 |
||||||||||||||||||
| Melting point |
172-174 ° C |
||||||||||||||||||
| boiling point |
460 ° C |
||||||||||||||||||
| solubility | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
1,3,5-Triphenylbenzene is a chemical compound from the group of benzene derivatives .
Extraction and presentation
1,3,5-Triphenylbenzene can be obtained by condensation of acetophenone using acids or by cyclopolymerization from phenylacetylene .
In the presence of traces of sodium methoxide, β-nitrostyrenes and two equivalents of dimethylformamide dimethylacetal in DMF form 1,3,5-triphenylbenzene in moderate yields (up to 40%).
properties
1,3,5-triphenylbenzene is a light brown solid that is practically insoluble in water. It has an orthorhombic crystal structure with the space group Pna 2 1 (space group no. 33) .
use
1,3,5-triphenylbenzene can be used to make other chemical compounds.
Individual evidence
- ↑ a b data sheet 1,3,5-triphenylbenzene, 99 +% from AlfaAesar, accessed on February 22, 2018 ( PDF )(JavaScript required) .
- ↑ a b c d e data sheet 1,3,5-triphenylbenzene, 97% from Sigma-Aldrich , accessed on February 21, 2018 ( PDF ).
- ↑ a b c d e William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, 2016, ISBN 978-1-4987-5429-3 , pp. 458 ( limited preview in Google Book search).
- ↑ Google Patents: DE1085864B - Process for the production of 1, 3, 5-triphenylbenzene by condensation of acetophenone using acids - Google Patents , accessed on February 22, 2018
- ^ Paul N. Rylander: Organic Syntheses with Noble Metal Catalysts . Elsevier, 2012, ISBN 978-0-323-16261-6 , pp. 330 ( limited preview in Google Book search).
- ↑ George Butler: Cyclopolymerization and Cyclocopolymerization . CRC Press, 1992, ISBN 978-0-8247-8625-0 , pp. 192 ( limited preview in Google Book search).
- ↑ TY Kim, HS Kim, KY Lee, JN Kim: N, N -dimethylformamide dimethylacetal (DMF-DMA) catalyzed formation of 1,3,5-trisubstituted benzene derivatives from α, β- unsaturated nitro compounds . In: Bull. Korean Chem. Soc. tape 20 , no. 11 , 1999, p. 1255–1256 ( PDF ( memento of October 26, 2017 in the Internet Archive )).
- ↑ MS Farag: The crystal structure of 1,3,5-triphenylbenzene. In: Acta Crystallographica . 7, 1954, pp. 117-121, doi: 10.1107 / S0365110X54000242 .
- ^ HO Wirth, W. Kern, E. Schmitz: Synthesis and properties of branched oligophenylenes, which are derived from 1,3,5-triphenylbenzene. 14th communication. In: The Macromolecular Chemistry . 68, pp. 69-99, doi: 10.1002 / macp.1963.020680106 .

