β-nitrostyrene
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| trans -β-nitrostyrene | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | β-nitrostyrene | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 7 NO 2 | ||||||||||||||||||
| Brief description |
light yellow to yellow crystalline powder or prismatic crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 149.15 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
55-58 ° C at 1.013 hPa |
||||||||||||||||||
| boiling point |
250-260 ° C |
||||||||||||||||||
| solubility |
practically insoluble in water, soluble in ethanol and acetone , very soluble in diethyl ether , chloroform and carbon disulfide |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
β-nitrostyrene is an aromatic nitro alkene , which mainly in the trans conformation is present and by a Henry reaction of benzaldehyde and nitromethane is easily accessible. As an α, β-unsaturated nitro compound , β-nitrostyrene is a suitable acceptor for enantioselective Michael additions to Michael donors, such as. B. aldehydes or ketones . β-Nitrostyrene is used in synthesis variants of the γ-aminobutyric acid derivatives baclofen and phenibut and as a precursor for phenethylamine and its derivatives.
Occurrence and representation
As early as 1839, when he distilled liquid Storax resin with concentrated nitric acid, Eduard Simon received an “essential oil” that smelled strongly of cinnamon and solidified in “splendid prisms”, which he called nitrostyrene.
August Wilhelm von Hofmann described and confirmed the substance obtained according to E. Simon's instructions as nitrostyrene.
The first targeted synthesis of β-nitrostyrene was reported in 1883 with the reaction of benzaldehyde with nitromethane in the presence of zinc chloride (acid catalysis).
Johannes Thiele presented β-nitrostyrene for the first time in 1899 in a nitroaldol condensation, later named the Henry reaction , by basic catalysis using potassium methoxide in "excellent yield" as yellowish prisms.
The nitro alcohol formed as an intermediate in the reaction of benzaldehyde with nitromethane dehydrates spontaneously with formation of the α, β-unsaturated nitroalkene.
With amylamine as the catalyst, Emil Knoevenagel obtained β-nitrostyrene in 75% yield.
A laboratory procedure that is simple in terms of preparation and using sodium hydroxide solution as a catalyst indicates a yield of 80 to 83%.
The use of methylamine or ammonium acetate in acetic acid as a catalyst has no advantages over alkali hydroxides or alcoholates.
The nitroaldol reaction in the ionic liquid 2- (hydroxyethyl) ammonium format gives small batches of β-nitrostyrene in 90% yield.
Nitration of styrene with nitrogen monoxide NO in 1,2-dichloroethane EDC and dehydration of the nitroalcohol that is formed at the same time by heating with acidic aluminum oxide gives very small batches (1 mmolar) β-nitrostyrene in 95% yield.
properties
β-Nitrostyrene is a yellow crystalline substance that crystallizes from ethanol in rhombic prisms when it cools and dissolves in many organic solvents. The compound "smells extremely like Zimm" and has "a sweet but extremely burning taste and a tear-irritating odor". The vapors from hot β-nitrostyrene solutions are extremely irritating to the nose and eyes; the skin of the face is particularly sensitive to the solid substance.
Applications
Hydrogenation to β-phenylethylamine
The complete hydrogenation of β-nitrostyrene with hydrogen on a palladium contact gives β-phenylethylamine in 84% yield, compared to 88% in the reduction with borane - tetrahydrofuran complex,
While hydrogenation with lithium aluminum hydride leads to phenylethylamine in only 60% yield, the parent compound is more pharmacologically effective, especially psychotropic phenylethylamines .
Diels-Alder reactions
As an activated alkene and dienophile , β-nitrostyrene reacts with dienes , e.g. B. 1,3-butadiene or 1,3-diphenylisobenzofuran in a Diels-Alder reaction with high yields to the corresponding adducts.
With cyclopentadiene a phenyl-2-nitro norbornene is formed in 85% yield .
Michael reactions
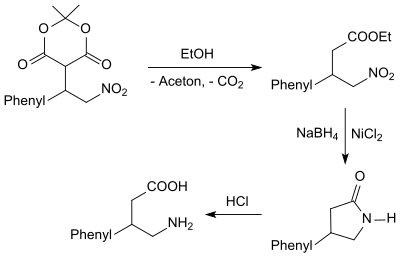
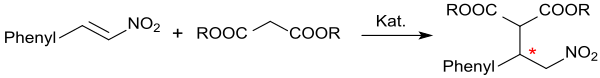
As an α, β-unsaturated nitro compound, β-nitrostyrene is an excellent acceptor in Michael additions. Β-Nitrostyrene reacts with Meldrum's acid in the presence of hydrotalcite in 95% yield to form the Michael addition product,
which when heated in ethanol with elimination of acetone and CO 2 in 85% yield 3-phenyl-4-nitrobutyric acid ethyl ester - a γ-nitrobutyric acid compound - forms. The nitrobutyric acid ester is converted into 4-phenylpyrrolidin-2-one (82%) with sodium borohydride in the presence of nickel (II) chloride hexahydrate, which is converted into the γ-aminobutyric acid (GABA) derivative 4-amino-3 with 6M hydrochloric acid -phenylbutyric acid (as hydrochloride ) phenibut is cleaved.
The same reaction sequence with 4-chloro-β-nitrostyrene as the starting compound gives the γ-aminobutyric acid derivative baclofen , which is effective as a muscle relaxant .
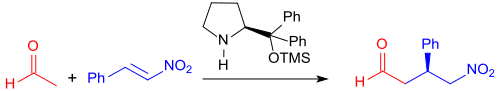
When using chiral catalysts, such as. B. quinine - alkaloids , prolinol derivatives or thiourea can with aldehydes - even with the weak nucleophile acetaldehyde
- and 1,3-dicarbonyl compounds - also with the sterically hindered dipivaloylmethane - Michael addition products can be represented in practically quantitative yield and enantiomeric excesses of up to 99%.
Baylis-Hillman reaction
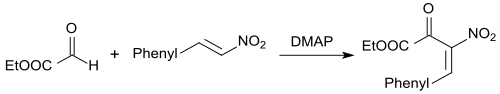
The Baylis-Hillman reaction is suitable for base-catalyzed CC linkage between aldehydes (or electrophiles) and activated alkenes in the presence of bases, such as. B. DABCO , with highly functionalized products such. B. α-substituted allyl alcohols arise. In the presence of diarylthioureas and the base DMAP , the aldehyde ethyl glyoxylate reacts with β-nitrostyrene in high yields to form 2-hydroxy-3-nitro-4-arylbut-3-enoates.
Barton-Zard reaction
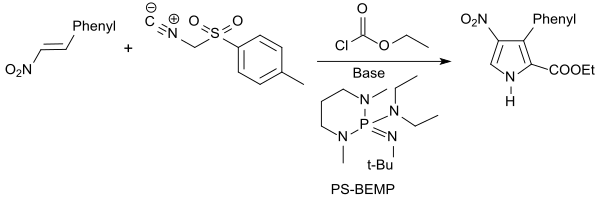
The Barton-Zard reaction named after Derek HR Barton , in which β-nitrostyrene (derivatives) with alkyl isocyanoacetates or tosylmethyl isocyanide TosMIC in the presence of a strong base, such as. B. react n -Butyllithium , produces substituted pyrroles. From β-nitrostyrene is formed with the isocyanide TosMIC and ethyl chloroformate the polymer-fixed in the presence of Super Base PS-BEMP, the 4-nitro-3-phenyl-1 H -pyrrole-2-ethyl carboxylate in 76% yield.
3,4-Diarylpyrroles are of interest as starting compounds for aryl-substituted porphyrins and can be prepared in a modest yield (approx. 50%) by reducing β-nitrostyrene with titanium (III) chloride in aqueous 1,4-dioxane , but prepared very easily become.
Further pyrrole syntheses using β-nitrostyrene (derivatives) are described.
Active ingredients based on β-nitrostyrene
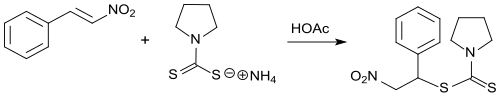
Addition products of dithiocarbamates to β-nitrostyrene were synthesized and tested for their effectiveness against bacteria and fungi.
β-Nitrostyrene and its halogen- or methyl-substituted derivatives were examined for antibacterial properties, as they show high reactivity in the Michael addition towards thiols from cysteine-rich enzymes. However, β-nitrostyrene itself is not very active.
In the presence of traces of sodium methoxide, β-nitrostyrenes and two equivalents of dimethylformamide dimethylacetal in DMF form 1,3,5-triphenylbenzenes in moderate yields (up to 40%) .
annotation
Β-nitrostyrene is sometimes incorrectly stated as a precursor for the dye indigo . However, Ludwig Gattermann mistakenly suspected 2-nitrostyrene as an intermediate stage with β-nitrostyrene.
Review article
- Conjugated nitroalkenes as intermediates
- Syntheses with conjugated nitroalkenes
- Advances in the Synthesis of Conjugated Nitroalkenes
Individual evidence
- ↑ Entry on trans-β-nitrostyrene at TCI Europe, accessed on October 25, 2017.
- ↑ a b c E. Simon: About the liquid Storax (Styrax liquidus) . In: Liebigs Ann. Chem. Band 31 , no. 3 , 1839, pp. 265-277 , doi : 10.1002 / jlac.18390310306 .
- ↑ a b J. Thiele: Condensation of nitromethane with aromatic aldehydes . In: Ber. German Chem. Ges. Volume 32 , no. 1 , 1899, p. 1293-1295 , doi : 10.1002 / cber.189903201209 .
- ↑ a b Data sheet trans-β-Nitrostyrene 99% from Sigma-Aldrich , accessed on October 25, 2017 ( PDF ).
- ↑ a b Data sheet trans-beta-Nitrostyrene, 98% from AlfaAesar, accessed on October 25, 2017 ( PDF )(JavaScript required) .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 91st Edition . CRC Press, Boca Raton, FL, USA 2010, ISBN 978-1-4398-2077-3 , pp. 3-396 .
- ↑ a b Data sheet β-nitrostyrene for synthesis (PDF) from Merck , accessed on October 25, 2017.
- ↑ C. Yu, W. Wang: Nitrostyrene . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2014, doi : 10.1002 / 047084289X.rn01748 .
- ^ A b N. Mase, K. Watanabe, H. Yoda, K. Takabe, F. Tanaka, CF Barbas: Organocatalytic direct Michael reaction of ketones and aldehydes with β-nitrostyrene . In: J. Am. Chem. Soc. tape 128 , no. 15 , 2006, p. 4966-4967 , doi : 10.1021 / ja060338e .
- ↑ a b C.RM D'Oca et al .: New Multicomponent Reaction for the Direct Synthesis of β-Aryl-γ-nitroesters Promoted by Hydrotalcite-Derived Mixed Oxides as Heterogeneous Catalyst . In: J. Braz. Chem. Soc. tape 28 , no. 2 , 2017, p. 285-298 , doi : 10.5935 / 0103-5053.20160175 .
- ↑ a b J. Blyth, AW Hofmann: Ueber das styrene and some of its products of decomposition . In: Liebigs Ann. Chem. Band 53 , no. 3 , 1845, p. 289-329 , doi : 10.1002 / jlac.18450530302 .
- ↑ B. Priebs: Effect of benzaldehyde on the mononitro hydrocarbons of the fat series . In: Ber. German Chem. Ges. Volume 16 , no. 2 , 1883, p. 2591 , doi : 10.1002 / cber.188201602191 .
- ↑ J. Thiele, S. Haeckel: Ueber Derivate des Phenylnitroäthylens . In: Liebigs Ann. Chem. Band 325 , no. 1 , 1902, pp. 1-18 , doi : 10.1002 / jlac.19023250102 .
- ^ E. Knoevenagel, L. Walter: Condensation of aliphatic nitro bodies with aromatic aldehydes by organic bases . In: Ber. German Chem. Ges. Volume 37 , no. 4 , 1904, pp. 4502-4510 , doi : 10.1002 / cber.19040370443 .
- ↑ a b D.E. Worrall: Nitrostyrene In: Organic Syntheses . 9, 1929, p. 66, doi : 10.15227 / orgsyn.009.0066 ; Coll. Vol. 1, 1941, p. 413 ( PDF ).
- ↑ CB Gairaud, GR Lappin: The synthesis of β-nitrostyrenes . In: J. Org. Chem. Band 18 , no. 1 , 1953, p. 1-3 , doi : 10.1021 / jo01129a001 .
- ↑ S. Pavlovica, A. Gaidule, A. Zicmanis: Synthesis of β-nitrostyrene in highly hydrophilic ionic liquid media . In: Latvian J. Chem. Volume 1 , 2013, p. 49-53 , doi : 10.2478 / ljc-2013-0005 .
- ↑ T. Mukaiyama, E. Hata, T. Yamada: Convenient and simple preparation of nitroolefins Nitration of olefins with nitric oxide . In: Chem. Lett. tape 24 , no. 7 , 1995, p. 505-506 , doi : 10.1246 / cl.1995.505 .
- ↑ K. Kindler, E. Brandt, E. Gehlhaar: Studies on the mechanism of chemical reactions. V. The importance of molecular compounds in catalytic hydrogenations . In: Liebigs Ann. Chem. Band 511 , no. 1 , 1934, p. 209-212 , doi : 10.1002 / jlac.19345110117 .
- ↑ GW Kabalka, LHM Guindi, RS Varma: Selected Reductions of conjugated nitroalkenes . In: Tetrahedron . tape 46 , no. 21 , 1990, pp. 7443-7457 , doi : 10.1016 / S0040-4020 (01) 89059-1 .
- ↑ RF Nystrom, WG Brown: Reduction of organic compounds by lithium aluminum hydride. III. Halides, quinones, miscellaneous nitrogen compounds . In: J. Am. Chem. Soc. tape 70 , no. 11 , 1948, pp. 3738-3740 , doi : 10.1021 / ja01191a057 .
- ↑ CFH Allen, A. Bell, JW Gates, Jr .: The diene synthesis with β-nitrostyrene . In: J. Org. Chem. Band 08 , no. 4 , 1943, pp. 373-379 , doi : 10.1021 / jo01192a011 .
- ↑ J. Bourguignon, G. Le Nard, G. Queguiner: Synthèse d'aryl nitrobornènes par cycloaddition de Diels-Alder entre les aryl-nitroéthylènes et le cyclopentadiène. Justification de la stéréochimie et de la réactivité relative observées par la methode CNDO / II. Obtention d'aryl aminonorbornènes . In: Can. J. Chem. Volume 63 , no. 9 , 1985, pp. 2354-2361 , doi : 10.1139 / v85-390 .
- ^ H. Li, Y. Wang, L. Tang, L. Deng: Highly enantioselective conjugate addition of malonate and β-ketoester to nitroalkenes: Asymmetric CC bond formation with new bifunctional organic catalysts based on cinchona alkaloids . In: J. Am. Chem. Soc. tape 126 , no. 32 , 2004, p. 9906-9907 , doi : 10.1021 / ja047281l .
- ^ D. Sarkar, R. Bhattarai, AD Headley, B. Ni: A novel recyclable organocatalytic system for the highly asymmetric Michael addition of aldehydes to nitroolefins in water . In: Synthesis . tape 12 , 2011, p. 1993-1997 , doi : 10.1055 / s-0030-1260465 .
- ↑ Enantio- and Diastereoselective Michael Reaction of 1,3-Dicarbonyl Compounds to nitroolefins Catalyzed by a Bifunctional Thiourea . In: J. Am. Chem. Soc. tape 127 , no. 1 , 2005, p. 119-125 , doi : 10.1021 / ja044370p .
- ^ P. Garciá-Garciá, A. Ladépeche, R. Halder, B. List: Catalytic asymmetric Michael reactions of acetaldehyde . In: Angew. Chem. Band 120 , no. 25 , 2008, p. 4797-4799 , doi : 10.1002 / anie.200800847 .
- ↑ Organocatalytic enantioselective Michael addition of β-diketones to β-nitrostyrene: The first Michael addition of dipivaloylmethane to an activated olefin; DP Gavin, JC Stephens, Arkivoc , 2011 (ix), 407-421 ( PDF .
- ↑ Patent DE2155113 : Process for the production of acrylic compounds. Filed November 5, 1971 , published May 10, 1972 , applicant: Celanese Corp., inventor: AB Baylis, MED Hillman.
- ↑ H.-H. Kuan, RJ Reddy, K. Chen: An efficient Morita-Baylis-Hillman reaction for the synthesis of multifunctional 2-hydroxy-3-nitrobut-3-enoate derivatives . In: Tetrahedron . tape 66 , no. 52 , 2010, p. 9875-9879 , doi : 10.1016 / j.tet.2010.10.061 .
- ↑ DHR Barton, SZ Zard: A new synthesis of nitroalkenes . In: J. Chem. Soc., Chem. Commun. 1985, p. 1098-1100 , doi : 10.1039 / C39850001098 .
- ↑ Barton-Zard Pyrrole Synthesis . In: Comprehensive Organic Name Reactions and Reagents . 2010, doi : 10.1002 / 9780470638859.conrr057 .
- ↑ IR Baxendale, CD Buckle, SV Ley, L. Tamborini: A base-catalysed one-pot three-component coupling reaction leading to nitrosubstituted pyrroles . In: Synthesis . tape 9 , 2009, p. 1485-1493 , doi : 10.1055 / s-0028-1087991 .
- ↑ SV Ley, JJ Scicinski: Schwesinger base, polymer-supported . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2002, doi : 10.1002 / 047084289X.rn00026 .
- ↑ N. Ono, H. Miyagawa, T. Ueta, H. Tani: Synthesis of 3,4-diarylpyrroles and conversion into dodecaarylporphyrins; a new approach to porphyrins with altered redox potential . In: J. Chem Soc, Perkin I.. . 1998, p. 1595-1601 , doi : 10.1039 / A801185K .
- ↑ V. Estévez, M. Villacampa, JC Menéndez: Multicomponent reactions for the synthesis of pyrroles . In: Chem. Soc. Rev. Band 39 , 2010, p. 4402-4421 , doi : 10.1039 / b917644f .
- ↑ Patent US4148795 : Dithiocarbamate ester bactericides and fungicides. Filed December 7, 1976 , published April 10, 1979 , Applicant: American Cyanamid Co., Inventor: TA Lies, JW Clapp.
- ↑ H. Cornell, T. Nguyen, G. Nicoletti, N. Jackson, H. Huegel : Comparisons of halogenated β-nitrostyrenes as antimicrobial agents . In: Appl. Sci. tape 4 , no. 3 , 2014, p. 380-389 , doi : 10.3390 / app4030380 .
- ^ N. Milhazes et al .: β-Nitrostyrene derivatives as potential antibacterial agents: A structure-property-activity relationship study . In: Bioorg. Med. Chem. Band 14 , no. 12 , 2006, p. 4078-4088 , doi : 10.1016 / j.bmc.2006.02.006 .
- ↑ TY Kim, HS Kim, KY Lee, JN Kim: N, N -dimethylformamide dimethylacetal (DMF-DMA) catalyzed formation of 1,3,5-trisubstituted benzene derivatives from α, β- unsaturated nitro compounds . In: Bull. Korean Chem. Soc. tape 20 , no. 11 , 1999, p. 1255-1256 ( easechem.com [PDF]). N, N -Dimethylformamide dimethylacetal (DMF-DMA) catalyzed formation of 1,3,5-trisubstituted benzene derivatives from α, β-unsaturated nitro compounds ( Memento of the original from October 26, 2017 in the Internet Archive ) Info: The archive link became automatic used and not yet tested. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ L. Gattermann, Laboratory Methods of Organic Chemistry , pp. 371-372, The Macmillan Co., New York, 1937.
- ↑ F. Sánchez-Viesca, M. Berros, R. Gómez: On the mechanism of the Baeyer-Drewsen synthesis of indigo . In: Amer. J. Chem. Volume 6 , no. 1 , 2016, p. 18-22 , doi : 10.5923 / j.chemistry.20160601.04 .
- ↑ AGM Barrett, GG Graboski: Conjugated nitroalkenes: Versatile intermediates in organic synthesis . In: Chem. Rev. Band 86 , no. 5 , 1986, pp. 751-762 , doi : 10.1021 / cr00075a002 .
- ↑ GW Kabalka, RS Varma: Syntheses and selected Reductions of conjugated nitroalkenes a review . In: Org. Prep. Proced. Int. tape 19 , no. 4-5 , 1987, pp. 283-328 , doi : 10.1080 / 00304948709356200 .
- ↑ G. Yan, AJ Borah, L. Wang: Recent advances in the synthesis of nitroolefin compounds . In: Org. Biomol. Chem. Band 12 , 2014, p. 6049-6058 , doi : 10.1039 / c4ob00573b .