Dimethylformamide dimethyl acetal
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
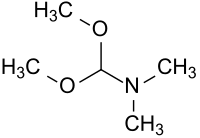
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dimethylformamide dimethyl acetal | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 13 NO 2 | |||||||||||||||
| Brief description |
clear, colorless to light yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 119.16 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.893 g cm −3 (20 ° C ) |
|||||||||||||||
| Melting point |
−85 ° C at 1.013 hPa |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.3972 (20 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Dimethylformamide dimethyl acetal is obtained by acetalization of dimethylformamide with methanol formed and two alkoxy groups at the carbonyl -bearing amide - acetal . The compound has become more widespread as a formylating reagent and methylating agent for carboxylic acids , phenols , amines , thiols and amino acids and as a building block , especially for heterocycles .
Occurrence and representation
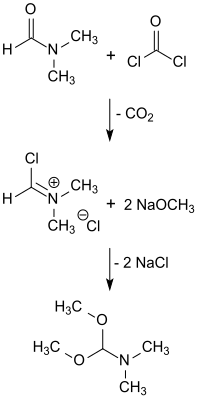
Analogously to the first publication on dimethylformamide diethylacetal from Hellmut Bredereck's group , the dimethylformamide dimethylacetal of the adduct obtained by reaction ( O - methylation ) of dimethylformamide with dimethyl sulfate with sodium methoxide in methanol at 0 ° C. is obtained in yields of 72 to 87%.
Because of the decomposition of DMF-DMA during distillation under normal pressure, rapid distillation of the reaction mixture with the addition of methanol as an entrainer and subsequent fractional distillation of the resulting methanol / DMF-DMA mixture is recommended. As a result, pure yields of dimethylformamide dimethylacetal of 85 to 90% are achieved.
The reaction of the Vilsmeier reagent N, N -dimethyl (chloromethylene) iminium chloride in chloroform with sodium methoxide in methanol gives DMF-DMA in 55% yield.
Dimethylformamide dimethylacetal is also formed when the reactants methanolate, DMF and CHCl 3 react . Chloroform reacts with solid sodium methoxide or sodium methoxide in methanol, presumably via the dichlorocarbene formed as an intermediate: CCl 2 , which converts dimethylformamide to DMF-DMA with elimination of CO.
In millimolar approaches, raw yields of up to 91% are achieved.
properties
Dimethylformamide-dimethyl acetal is a clear, colorless, amine-like smelling liquid that mixes with water and many organic solvents. The acetal gradually decomposes in water.
Applications
DMF-DMA as a methylating agent
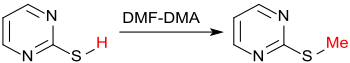
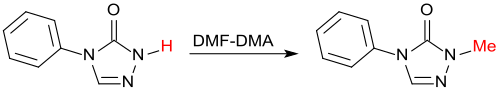
Acid compounds such as B. carboxylic acids, phenols, thiols are methylated by dimethylformamide dimethylacetal in a smooth reaction,
as well as NH-heterocycles, such as. B. Triazoles .
DMF-DMA as a formylating agent
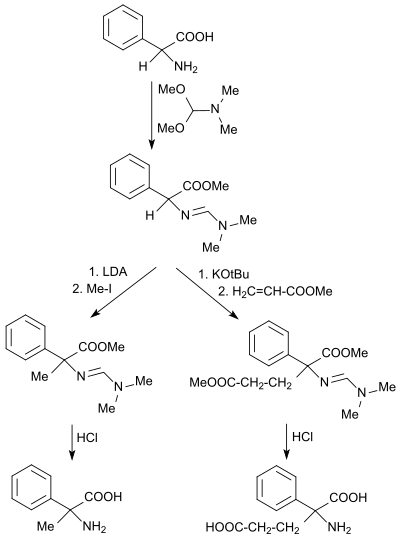
Dimethylformamide dimethylacetal reacts with primary amines to give N, N-dimethylformamidines and with α- amino acids to give the corresponding, relatively stable α-formamidinomethyl esters.
The α-formamidinomethyl ester can with good yields after deprotonation with strong bases, such as. B. lithium diisopropylamide LDA or potassium tert-butoxide KOtBu with alkyl halides , such as. B. iodomethane or alkylated in a Michael addition with z. B. methyl acrylate are implemented.
As an alternative to the synthesis of the cyclic polyamine Cyclen according to Reed and Weisman, which is more suitable as a laboratory process , the reaction of triethylenetetramine TETA with DMF-DMA to form the bis-amidine 1,1'-ethylenedi-2-imidazoline in 85% yield is suitable (1 . Step). Its macrocyclization under dilution conditions with 1,2-dibromoethane gives a monoimidazolium compound in 70% yield, which is then cleaved with boiling potassium hydroxide in a yield of 88% for cycling.
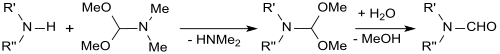
The corresponding N -formyl- N, N- dialkylamines are formed by reaction of dimethylformamide dimethylacetal with secondary amines .
With CH-acidic compounds with activated methylene groups, such as. B. ketones , DMF-DMA reacts smoothly to vinylogous amides, the so-called enaminones.
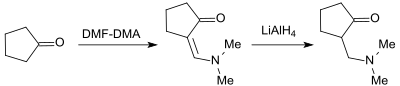
Cyclopentanone, for example, forms the corresponding enaminone with DMF-DMA in 86% yield, which, with lithium aluminum hydride LiAlH 4, gives the corresponding Mannich base in 88% yield .
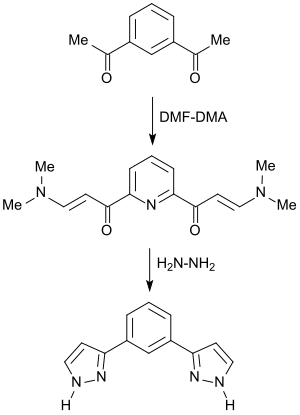
With diketones , bis-enaminones are formed analogously, from which a large number of heterocycles are accessible.
The bis-enaminone, which is formed almost quantitatively in the reaction of 2,6-diacetylpyridine with DMF-DMA, reacts with hydrazine to form 3-arylpyrazole.
An effective method for the preparation of substituted indoles is what is known in the English literature as the “Leimgruber-Batcho indole synthesis” from 2-nitrotoluenes and dimethylformamide dimethylacetal, pyrrolidine being added to accelerate the reaction .
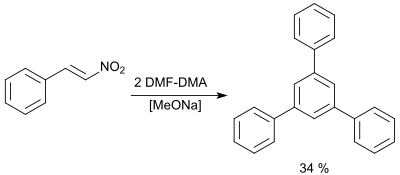
In the presence of traces of sodium methoxide, β-nitrostyrenes and two equivalents of DMF-DMA in DMF form 1,3,5-triphenylbenzenes in moderate yields (up to 40%) .
Other reactions with DMF-DMA
Like the homologous dimethylformamide diethylacetal, DMF-DMA can be transacetalised with excess higher alcohols to give the corresponding dimethylformamide dialkyl acetals ( transacetalisation ).
The transamidation of DMF-DMA with dibenzylamine yields N, N -dibenzylformamide dimethylacetal, which is suitable as a protecting group for primary amines, with which it reacts smoothly to form amidines . This protective group is stable to acids, bases and nucleophiles and can be split off again by catalytic hydrogenation with palladium (II) oxide on activated carbon .
Dimethylformamide dimethylacetal can be used as a scavenger for hydrogen sulfide H 2 S and mercaptans in natural gas and hydrocarbon-based fuels, such as. B. gasoline, diesel or kerosene can be used.
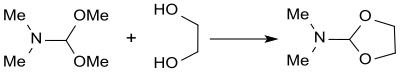
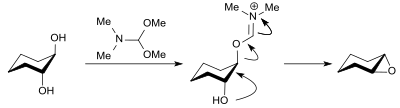
With 1,2- diols , such as. B. trans -cyclohexane-1,2-diol, DMF-DMA reacts in high yield (88%) with reversal of configuration to the epoxide cyclohexene oxide .
Individual evidence
- ↑ Entry on N, N-Dimethylformamide Dimethyl Acetal for Esterification at TCI Europe, accessed on September 25, 2017.
- ↑ a b c data sheet N, N-dimethylformamide-dimethylacetal from Sigma-Aldrich , accessed on September 25, 2017 ( PDF ).
- ↑ a b c data sheet N, N-Dimethylformamid dimethyl acetal, 97% from AlfaAesar, accessed on September 25, 2017 ( PDF )(JavaScript required) .
- ↑ a b c d e f Data sheet N, N-dimethylformamide dimethylacetal for synthesis (PDF) from Merck , accessed on September 25, 2017.
- ↑ a b R.F. Abdulla, RS Brinkmeyer: The chemistry of formamide acetals . In: Tetrahedron . tape 35 , no. 14 , 1979, pp. 1675-1735 , doi : 10.1016 / 0040-4020 (79) 88001-1 .
- ↑ a b c U. Pindur: N, N-Dimethylformamide Diethyl Acetal . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2001, doi : 10.1002 / 047084289X.rd336 .
- ↑ D. Kidjemet: N, N-Dimethylformamide Dimethyl Acetal . In: Synlett . tape 10 , 2002, p. 1741-1742 , doi : 10.1055 / s-2002-34251 .
- ↑ FA Abu-Shabab, SM Sherif, SAS Mousa: Dimethylformamide dimethyl acetal as a building block in heterocyclic synthesis . In: J. Heterocyclic Chem. Volume 46 , no. 5 , 2009, p. 801-827 , doi : 10.1002 / jhet . 69 .
- ↑ H. Bredereck, F. Effenberger, G. Simchen: Reactive acid amide dimethyl sulfate complexes . In: Angew. Chem. Band 73 , no. 14 , 1961, pp. 493 , doi : 10.1002 / anie.19610731407 .
- ↑ H. Bredereck, G. Simchen, S. Rebsdat, W. Kantlehner, P. Horn, R. Wahl, H. Hoffmann, P. Grieshaber: acid amide reactions, L; Orthoamides, I Representation and properties of the amide acetals and aminal esters . In: Chem. Ber. tape 101 , no. 1 , 1964, pp. 41-50 , doi : 10.1002 / cber.19681010108 .
- ↑ D. Mesnard, L. Miginiac: Synthèse d'amine régiospécifique tertiaries à groupe secondaires bi-insaturé . In: J. Organometallic Chem. Volume 373 , no. 1 , 1989, pp. 1-10 , doi : 10.1016 / 0022-328X (89) 85018-1 .
- ↑ Patent DE2215954 : Process for the distillative preparation of dimethylformamide dimethylacetal. Registered on April 1, 1972 , published on November 23, 1972 , applicant: Deutsche Akademie der Wissenschaften zu Berlin, inventor: H. Groß, L. Haase, I. Keitel.
- ↑ H. Eilingsfeld, M. Seefelder, H. Weidinger: Amidchloride und Carbamidchloride . In: Angew. Chem. Band 72 , no. 22 , 1960, pp. 836-845 , doi : 10.1002 / anie.19600722208 .
- ↑ H. Eilingsfeld, M. Seefelder, H. Weidinger: Syntheses with amide chlorides, I. Reactions on the functional group N, N-disubstituted carboxamide chlorides . In: Chem. Ber. tape 96 , no. 10 , 1963, pp. 2671-2690 , doi : 10.1002 / cber.19630961023 .
- ^ JW Scheeren, RJF Nivard: Synthesis and stability of tri-sec-aminomethanes . In: Recl. Trav. Chim. Pays-Bas . tape 88 , no. 3 , 1969, p. 289-300 , doi : 10.1002 / recl.19690880306 .
- ↑ PL Anelli, M. Brocchetta, D. Copez, D. Palano, MVP Paoli: Unexpected formation of acylformamidines by reaction of primary carboxamides with MeONa in DMF in the presence of CHCl 3 . In: Tetrahedron . tape 53 , no. 46 , 1997, pp. 15827-15832 , doi : 10.1016 / 0040-4020 (97) 10041-2 .
- ↑ J. Gloede, B. Costisella: On the reaction of dimethylformamide dimethylacetal with acidic compounds . In: J. Prakt. Chem. Band 313 , no. 2 , 1971, p. 277-286 , doi : 10.1002 / prac.19713130212 .
- ↑ a b H. Vorbrüggen: The reaction of carboxylic acids and phenols with amide acetals . In: Angew. Chem. Band 75 , no. 6 , 1963, pp. 296-297 , doi : 10.1002 / anie.19630750612 .
- ↑ J.-P. Thenot, EC Horning, M. Stafford: Fatty acid esterification with N, N-dimethylformamide dialkyl acetals for GC analysis . In: Analyt. Lett. tape 5 , no. 4 , 1972, p. 217-2223 , doi : 10.1080 / 00032717208069552 .
- ↑ A. Holý: Transformation of nucleosides into their 5'-deoxy derivatives . In: Tetrahedron Lett. tape 13 , no. 7 , 1972, p. 585-588 , doi : 10.1016 / S0040-4039 (01) 84384-7 .
- ↑ G. Fairley, C. Hall, R. Greenwood: Selective Methylation of NH-Containing Heterocycles and Sulfonamides Using N, N-Dimethylformamide Dimethylacetal Based on Calculated pK a Measurements . In: Synlett . tape 24 , no. 5 , 2013, p. 570-574 , doi : 10.1055 / s-0032-1318315 .
- ^ DA Dickman, M. Boes, AI Meyers: (S) -N, N-dimethyl-N- (1-tert-butoxy-3-methyl-2-butyl) formamidine In: Organic Syntheses . 67, 1989, p. 52, doi : 10.15227 / orgsyn.067.0052 ; Coll. Vol. 8, 1993, p. 204 ( PDF ).
- ↑ JJ Fitt, HW Gschwend: α-alkylation and Michael addition of amino acids: a practical method . In: J. Org. Chem. Band 42 , no. 15 , 1977, pp. 2639-2641 , doi : 10.1021 / jo00435a027 .
- ^ DP Reed, GR Weisman: 1,4,7,10-Tetraazacyclododecane In: Organic Syntheses . 78, 2002, p. 73, doi : 10.15227 / orgsyn.078.0073 ; Coll. Vol. 10, 2004, p. 667 ( PDF ).
- ↑ PS Athey, GE Kiefer: A new, facile synthesis of 1,4,7,10-tetraazacyclododecane: Cyclen . In: J. Org. Chem. Band 67 , no. 12 , 2002, p. 4081-4085 , doi : 10.1021 / jo016111d .
- ↑ Patent US5587451A : Process for preparing polyazamacrocycles. Filed October 1, 1994 , published December 24, 1996 , Applicant: The Dow Chemical Co., Inventor: PS Athey, GE Kiefer.
- ↑ J.-P. Thenot, TI Ruo, OJ Bouwsma: Formylation of secondary amines with dimethylformamide dimethylacetal . In: Analyt. Lett. tape 13 , no. 9 , 1980, pp. 759-769 , doi : 10.1080 / 00032718008077997 .
- ↑ A.-ZA Elassar, AA El-Khair: Recent developments in the chemistry of enaminones . In: Tetrahedron . tape 59 , no. 43 , 2003, p. 8463-8480 , doi : 10.1016 / S0040-4020 (03) 01201-8 .
- ↑ PF Schuda, CB Ebner, TM Morgan: The synthesis of Mannich bases from ketones and esters via enaminones . In: Tetrahedron Lett. tape 27 , no. 23 , 1986, pp. 2567-2570 , doi : 10.1016 / S0040-4039 (00) 84586-4 .
- ↑ Bis-enaminones as versatile precursors for terheterocycles: synthesis and reactions; AS Shawali, Arkivoc , 2012 (i), 383-431 ( PDF ).
- ↑ A.-K. Pleier, H. Glas, M. Grosche P. Sirsch, WR Thiel: Microwave-assisted synthesis of 1-aryl-3-dimethylaminoprop-2-enones: A simple and rapid access to 3 (5) -aryl pyrazoles . In: Synthesis . tape 1 , 2001, p. 55-62 , doi : 10.1055 / s-2001-9761 .
- ↑ AD Batcho, W. Leimgruber: Indoles from 2-methylnitrobenzenes by condensation with formamide acetals followed by reduction: 4-benzyloxyindole In: Organic Syntheses . 63, 1985, p. 214, doi : 10.15227 / orgsyn.063.0214 ; Coll. Vol. 7, 1990, p. 34 ( PDF ).
- ↑ TY Kim, HS Kim, KY Lee, JN Kim: N, N -dimethylformamide dimethylacetal (DMF-DMA) catalyzed formation of 1,3,5-trisubstituted benzene derivatives from α, β- unsaturated nitro compounds . In: Bull. Korean Chem. Soc. tape 20 , no. 11 , 1999, p. 1255-1256 ( easechem.com [PDF]). N, N -Dimethylformamide dimethylacetal (DMF-DMA) catalyzed formation of 1,3,5-trisubstituted benzene derivatives from α, β-unsaturated nitro compounds ( Memento of the original from October 26, 2017 in the Internet Archive ) Info: The archive link became automatic used and not yet tested. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ H. Meerwein, W. Florian, N. Schön, G. Stopp: About acid amide acetals, urea acetals and lactam acetals . In: Justus Liebigs Ann. Chem. Band 641 , no. 1 , 1961, pp. 1-39 , doi : 10.1002 / cber.19616410102 .
- ^ S. Vincent, S. Mons, L. Lebeau, C. Mioskowski: N, N-Dibenzyl formamidine as a new protective group for primary amines . In: Tetrahedron Lett. tape 38 , no. 43 , 1997, pp. 7527-7530 , doi : 10.1016 / S0040-4039 (97) 10023-5 .
- ↑ Patent US9273254B2 : Amino acetals and ketals as hydrogen sulfide and mercaptan scavengers. Filed December 20, 2013 , published March 1, 2016 , applicant: Ecolab USA Inc., inventor: DR Compton, K. Ekoue-Kovi.